CBSE Class 9 Answered
State the relationship between the atoms given below. Define the term used for this relationship. Write one characteristic feature of such atoms.
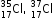
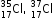
Asked by Topperlearning User | 16 Mar, 2015, 05:21: PM
The two atoms represent isotopes of chlorine. They have the same atomic number but different mass number. One characteristic feature:
They have same chemical properties.
Answered by | 16 Mar, 2015, 07:21: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by namyairmalwar9803.5sdatl | 07 Jun, 2020, 01:47: PM
CBSE 9 - Chemistry
Asked by pradoshsanesar | 10 Jan, 2020, 12:01: PM
CBSE 9 - Chemistry
Asked by maltidevi8800 | 06 Dec, 2019, 09:29: PM
CBSE 9 - Chemistry
Asked by ABHILASHA | 22 Aug, 2019, 08:46: AM
CBSE 9 - Chemistry
Asked by dubeykrishna49025 | 14 Oct, 2018, 10:02: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:05: PM









