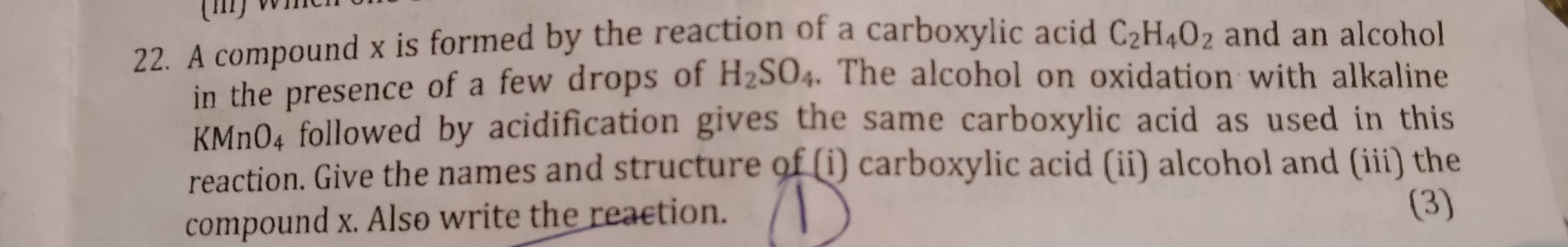CBSE Class 10 Answered
State the principle on which the cleansing action of soap is based.
Asked by Jitendra | 08 Jan, 2019, 07:59: PM
- Soap on mixing with water forms a concentrated solution and causes foaming.
- The long non-polar end of soap gravitates towards and surrounds the dirt and absorbs the dust in it.
- The short polar end with the carboxylate ion repels the water away from the dirt.
- A spherical aggregate of soap molecules is formed in the soap solution in water and is called a micelle.
- Thus, the soap molecule dissolves the dirt and our clothes get clean.
Answered by Ramandeep | 09 Jan, 2019, 10:00: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 07:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 03:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 11:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 08:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 05:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 10:07: PM