CBSE Class 9 Answered
State the postulates put forward by Neil's Bohr about the model of an atom. Draw a diagram to show the arrangement of energy levels in an atom.
Asked by Topperlearning User | 22 Jan, 2015, 03:38: PM
In 1913 Neils Bohr put forward the following postulates about the model of an atom:
- Electrons revolve around the nucleus only in certain permissible circular orbits. This orbit is called shell. The first shell from the nucleus is 'K' shell The subsequent shells are L, M, N, 0,..respectively.
- Electrons in each shell have been associated with definite amount of energy. Electrons in higher shell have more energy than those nearer to the nucleus.
- The energy of an electron remains constant as long as it revolves in its own shell. The shells are also called energy levels or stationary energy levels.
- According to Bohr's model, electrons occupy certain stable orbits or shells. Each shell has definite energy.
- These orbits or shells are represented by the letters K, L, M, N, …. or the numbers 1, 2, 3, 4, …
- The maximum number of electrons present in the shell is given by the formula (2n2), where n is the orbit number or shell number.
- The maximum numbers of electrons in different shells are as follows:
- The first orbit or K shell will have 2 × 12 = 2 electrons.
- The second shell will have 2 × 22 = 8 electrons.
- The third shell will have 2 × 32 = 18 electrons.
- The fourth shell will have 2 × 42 = 32 electrons and so on.
Electrons are not accommodated in a given shell unless the inner shells are filled.
Diagram to show the arrangement of energy levels:
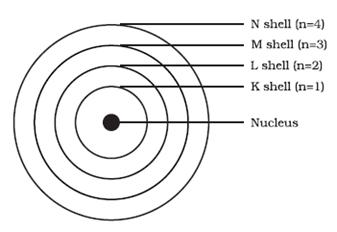
Answered by | 22 Jan, 2015, 05:38: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by archanakad | 25 Aug, 2020, 08:59: PM
CBSE 9 - Chemistry
Asked by Jaiwanthsiva | 15 Apr, 2020, 01:51: PM
CBSE 9 - Chemistry
Asked by Jaiwanthsiva | 12 Apr, 2020, 11:47: AM
CBSE 9 - Chemistry
Asked by snaik0856 | 29 Dec, 2019, 09:47: PM
CBSE 9 - Chemistry
Asked by Regiesmalom99 | 18 Oct, 2019, 01:36: PM
CBSE 9 - Chemistry
Asked by Vishalgarg1234 | 25 Nov, 2018, 03:21: PM
CBSE 9 - Chemistry
Asked by hk.sachdev | 12 Oct, 2018, 10:38: PM
CBSE 9 - Chemistry
Asked by sshivali3333 | 19 Sep, 2018, 11:06: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 12:49: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 12:50: PM







 . Then i have to first react Carbon with Oxygen then react that compond with calcium. So how will i know the valency of compound formed from reaction of carbon with oxygen. We can know the valency of elements from their atomic number and electronic configuration, but how can i know the valency of compound?
Please help,waiting for your reply,
Thank you.
. Then i have to first react Carbon with Oxygen then react that compond with calcium. So how will i know the valency of compound formed from reaction of carbon with oxygen. We can know the valency of elements from their atomic number and electronic configuration, but how can i know the valency of compound?
Please help,waiting for your reply,
Thank you. 