CBSE Class 11-science Answered
State postulate of kinetic molecular
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
Kinetic Molecular Theory of Gases:
Assumptions or postulates of kinetic molecular theory of gases:
- Gases consists of large number of minute identical particles (atoms or molecules) which are very small.
- Gas molecules are so far apart from each other that the actual volume of the molecules is negligible as compared to the total volume of gas. They are thus considered as point masses.
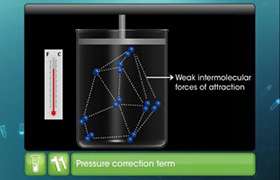
- There is no force of attraction between the particles of a gas at ordinary temperature and pressure.
- Particles of a gas are always in constant and random motion.
- Particles of a gas move in all possible directions in straight lines. During their random motion, they collide with each other and with the walls of the container.
- Pressure is exerted by the gas as a result of collision of the particles with the walls of the container.
- Collisions of gas molecules are perfectly elastic i.e., the total energy of molecules before and after the collision remains a same.
- There can exchange of energy between colliding molecules, their individual energies may change, but the sum of their energies will remain constant.
- At any particular time, different particles in the gas have different speeds and hence different kinetic energies.
- In kinetic theory, it is assumed that the average kinetic energy of the gas molecules is directly proportional to the absolute temperature.
Answered by Varsha | 18 Oct, 2018, 10:20: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 26 Jan, 2019, 12:57: PM
CBSE 11-science - Chemistry
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:19: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM