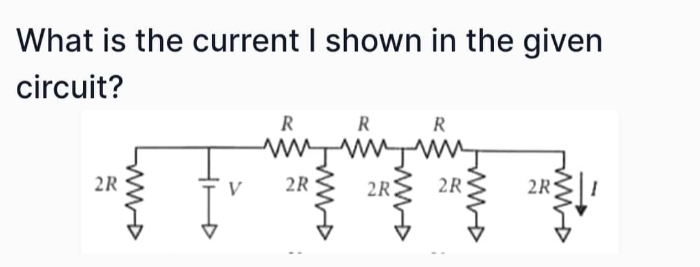
JEE Class main Answered
Solve

Asked by sarveshvibrantacademy | 14 May, 2019, 09:57: AM
Let us assume the initial state of 1 mole of ideal monoatomic gas as pressure P0 , Volume V0 and Temperature T0
At Initial state, Internal energy of ideal monoatomic gas = (3/2)nRT0 ..............(1)
where n is number of moles, R is universal gas constant. Hence we have (3/2)RT0 = 18000 J ..................(2)
The gas undergoes intermediate state by isochoric process (volume constant) so that intermediate state pressure is P0/3.
For ideal gas, when volume is constant, Pressure is proportional to Temperature.
Hence in Intermediate state Temperature becomes T0/3 .
Hence internal energy at intermediate state = (3/2)R(T0/3) = 18000/3 = 6000 J ...................(3)
[ eqn.(2) is used in eqn.(3) to get internal energy at intermediate state ]
Change in internal energy dU from initial state to intermediate state = 6000 -18000 = -12000 J
Since volume is constant in isochoric process, workdone dw = 0 and Heat released dQ to cool from
temperature T0 to (T0 /3 ) is equal to change in internal energy du
Hence from initial state to intermediate state, dQ = du = -12000 J ...............................(4)
Now the gas undergoes isobaric process (pressure constant) to reach a final state so that
final state temeperature is same as Initial state temperature T0 .
For ideal gas, when pressure is constant, volume is proportional to temperature.
From intermediate state to final state, temperature changes from (T0/3) to T0 .
Hence from intermediate state to final state,Volume changes from V0 to 3V0 . ( V/T is constant)
Workdone dW by the gas is given by, dW = (P0 /3) [ 3V0 - V0 ] = (2/3)P0V0 = (2/3) RT0 .......................(5)
using eqn.(2) and eqn.(5), we get dW = 8000 J ........................(6)
when the gas reaches final state, temperature of final state is same as that of initial state, i.e., T0
Since internal energy is related only to temperature and number of moles, final state internal energy = 18000 J.
Hence change in internal energy dU from intermediate state to final state is given by, dU = 18000 - 6000 = 12000 J
From first law of thermodynamics, we get the absorbed heat dQ as given by ,
dQ = dU + dW = 12000 +8000 = 20000 J ..........................(7)
from eqn.(4) and eqn.(7), net amount of heat energy absorbed = -12000 + 20000 = 8000 J
Answered by Thiyagarajan K | 14 May, 2019, 09:29: PM
Application Videos
JEE main - Physics
Asked by arivaryakashyap | 23 Apr, 2024, 10:40: AM
JEE main - Physics
Asked by ratnadeep.dmr003 | 21 Apr, 2024, 11:06: PM
JEE main - Physics
Asked by ksahu8511 | 19 Apr, 2024, 11:55: AM
JEE main - Physics
Asked by mohammedimroz | 13 Apr, 2024, 09:48: PM
JEE main - Physics
Asked by medhamahesh007 | 02 Apr, 2024, 11:11: AM
JEE main - Physics
Asked by gundlasumathi93 | 31 Mar, 2024, 02:13: PM
JEE main - Physics
Asked by chhayasharma9494 | 31 Mar, 2024, 12:47: PM
JEE main - Physics
Asked by archithateja3 | 30 Mar, 2024, 10:23: PM
JEE main - Physics
Asked by Machinenineha | 27 Mar, 2024, 05:28: PM