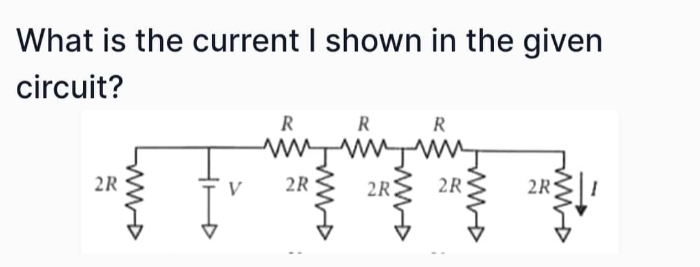
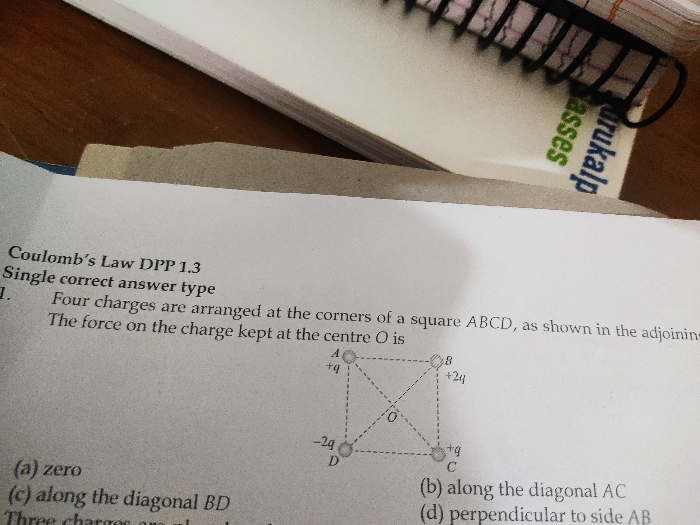JEE Class main Answered
solve
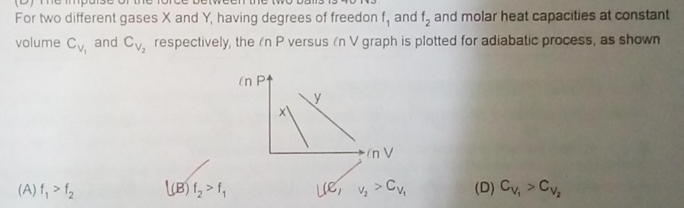
Asked by adjacentcalliber10 | 10 May, 2019, 12:00: PM
for adiabatic process, we have PV γ = K ...................(1)
where P is pressure, V is volume, γ is ratio of specific heat of constant volume and constant pressure and K is a constant.
by taking logarithm on both sides of eqn.(1), we have ln(P) + γ ln(V) = ln(K)
Hence we have, ln(P) = ln(K) - γ ln(V) ..............................(2)
Eqn.(2) shows the linear relationship between ln(P) and ln(V) as shown in graphs of figure given in question with negative slope.
Hence slope of each graph equals the ratio of specific heat of constant volume and constant pressure.
slope of graph-X that represent gas-1 is greater than slope of graph-Y that represent gas-2.
Hence ratio of specific heats of gas-1 is graeter than that of gas-2
γ1 > γ2 ;
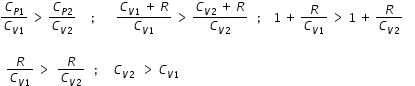
Specific heat is directly prportional to degrees of freedom f . Since we have CV2 > CV1 , then f2 > f1
Answered by Thiyagarajan K | 12 May, 2019, 05:24: PM
Application Videos
JEE main - Physics
Asked by arivaryakashyap | 23 Apr, 2024, 10:40: AM
JEE main - Physics
Asked by ratnadeep.dmr003 | 21 Apr, 2024, 11:06: PM
JEE main - Physics
Asked by ksahu8511 | 19 Apr, 2024, 11:55: AM
JEE main - Physics
Asked by mohammedimroz | 13 Apr, 2024, 09:48: PM
JEE main - Physics
Asked by medhamahesh007 | 02 Apr, 2024, 11:11: AM
JEE main - Physics
Asked by gundlasumathi93 | 31 Mar, 2024, 02:13: PM
JEE main - Physics
Asked by chhayasharma9494 | 31 Mar, 2024, 12:47: PM
JEE main - Physics
Asked by archithateja3 | 30 Mar, 2024, 10:23: PM
JEE main - Physics
Asked by Machinenineha | 27 Mar, 2024, 05:28: PM