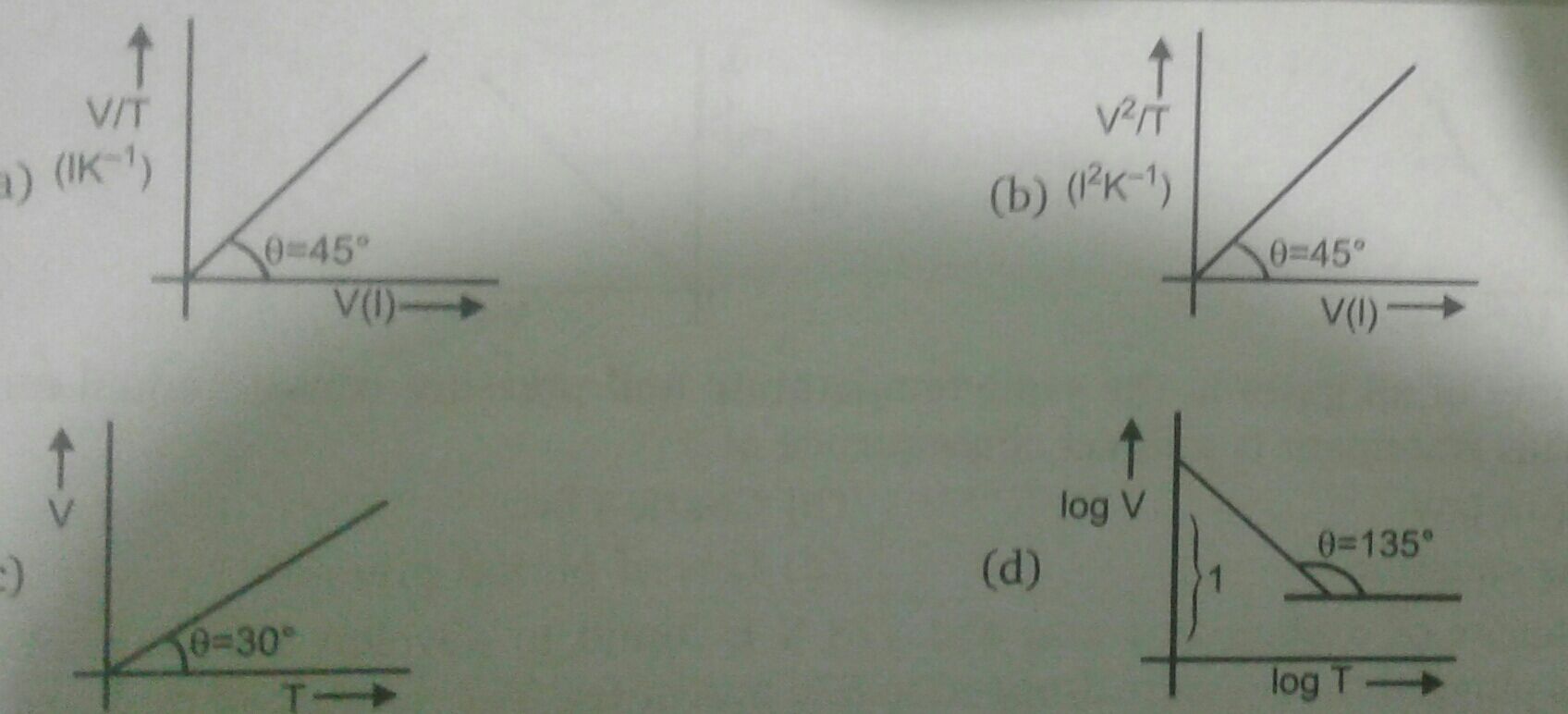JEE Class main Answered
Sir plz solve it step by step ,U may leave part (b) but plz solve explaining part(a)

Asked by vishakhachandan026 | 08 Aug, 2019, 10:21: AM
Given:
For NO,
Volume = 0.25 L
Pressure = 800 torr
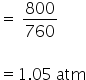
temperature = 220 K
By using gas equation, no. of moles can be calculated as,
PV = nRT
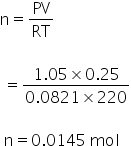
For O2
For NO,
Volume = 0.1 L
Pressure = 600 torr
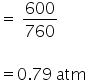
temperature = 220 K
By using gas equation, no. of moles can be calculated as,
PV= nRT
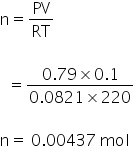
The reaction to form N2O4
2NO + O2 → N2O4
2 mole of NO reacts with 1 mole of O2
so, 0.00437 mole of O2 will require
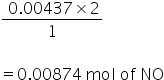
(a)
Excess of NO is : 0.0.145 - 0.00874 = 0.00578 mole of NO
Now aain by using gas law we can calculate pressure,
PV = nRT

(b) From equation,
2 mole of NO gives 1 mole of N2O4
so 0.00874 mole of NO will give,
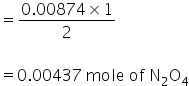
Molar mass of N2O4 = 92g/mol
Mass of N2O4 = 92×0.00437
= 0.402 gm.
Answered by Varsha | 08 Aug, 2019, 12:52: PM
JEE main - Chemistry
Asked by ruchisharmatbn | 06 Apr, 2024, 08:42: AM
JEE main - Chemistry
Asked by arjuns94037 | 07 Jan, 2024, 12:43: PM
JEE main - Chemistry
Asked by vishakhachandan026 | 08 Aug, 2019, 10:21: AM
JEE main - Chemistry
Asked by ashutosharnold1998 | 08 Aug, 2019, 12:11: AM
JEE main - Chemistry
Asked by vishakhachandan026 | 04 Jul, 2019, 02:59: PM
JEE main - Chemistry
Asked by Anish | 14 Mar, 2019, 11:14: AM
JEE main - Chemistry
Asked by Anish | 09 Jan, 2019, 11:33: PM