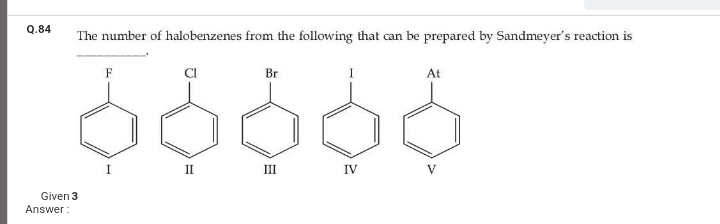
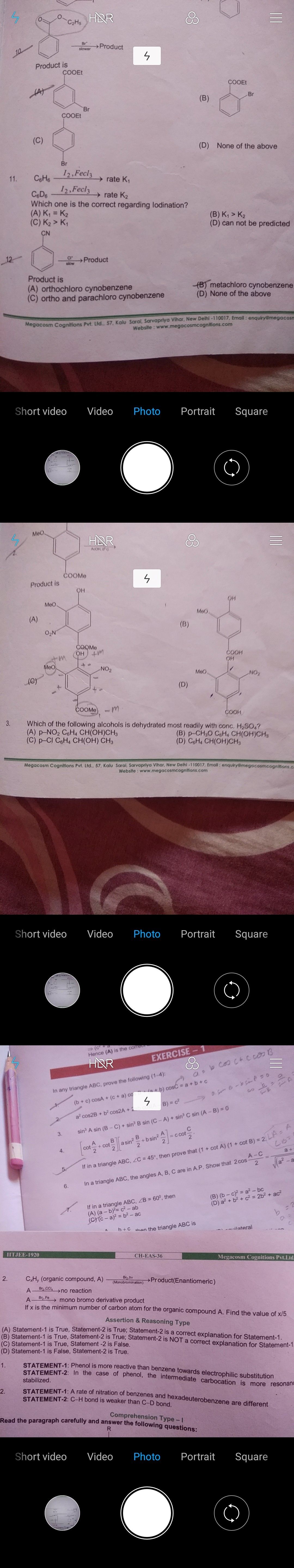
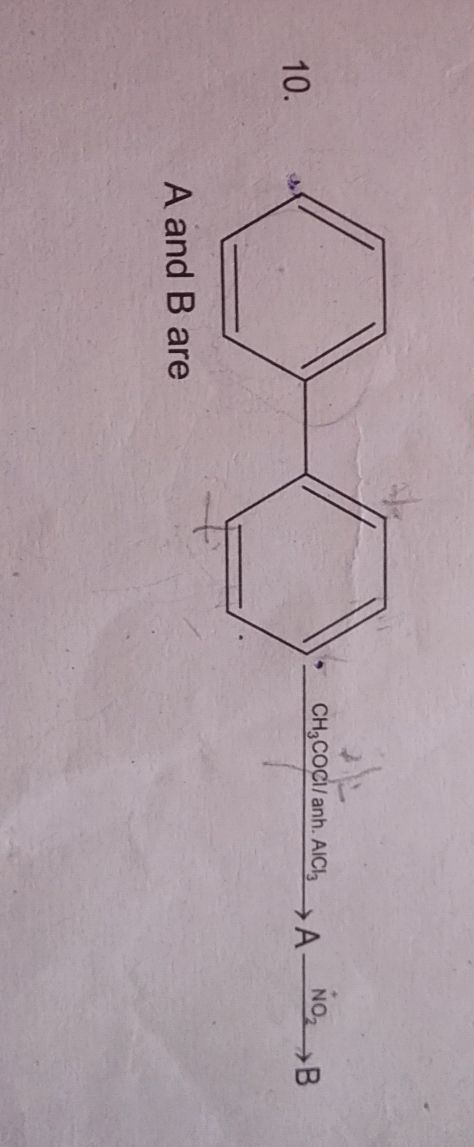
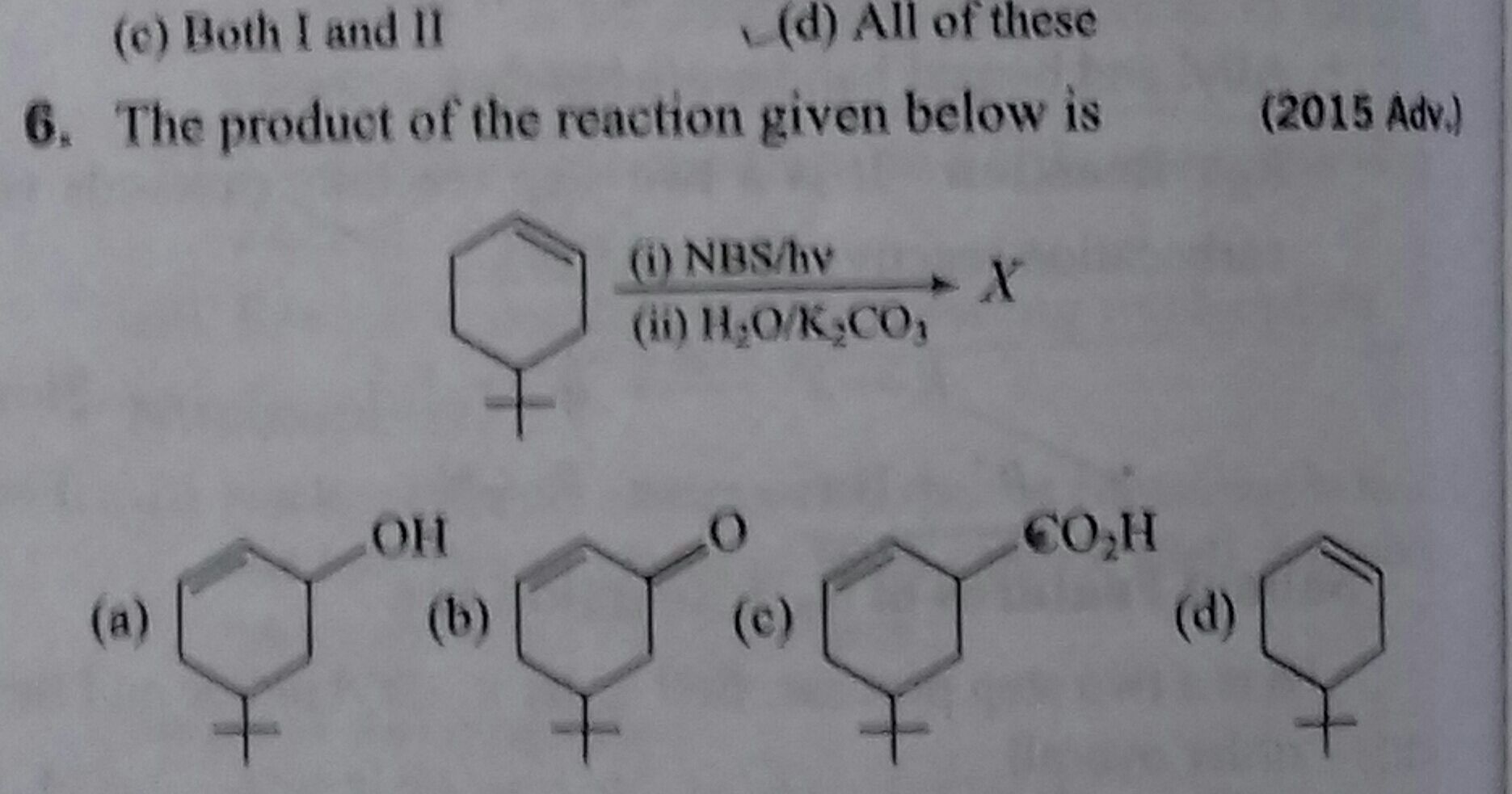JEE Class main Answered
Sir, pls solve the following.
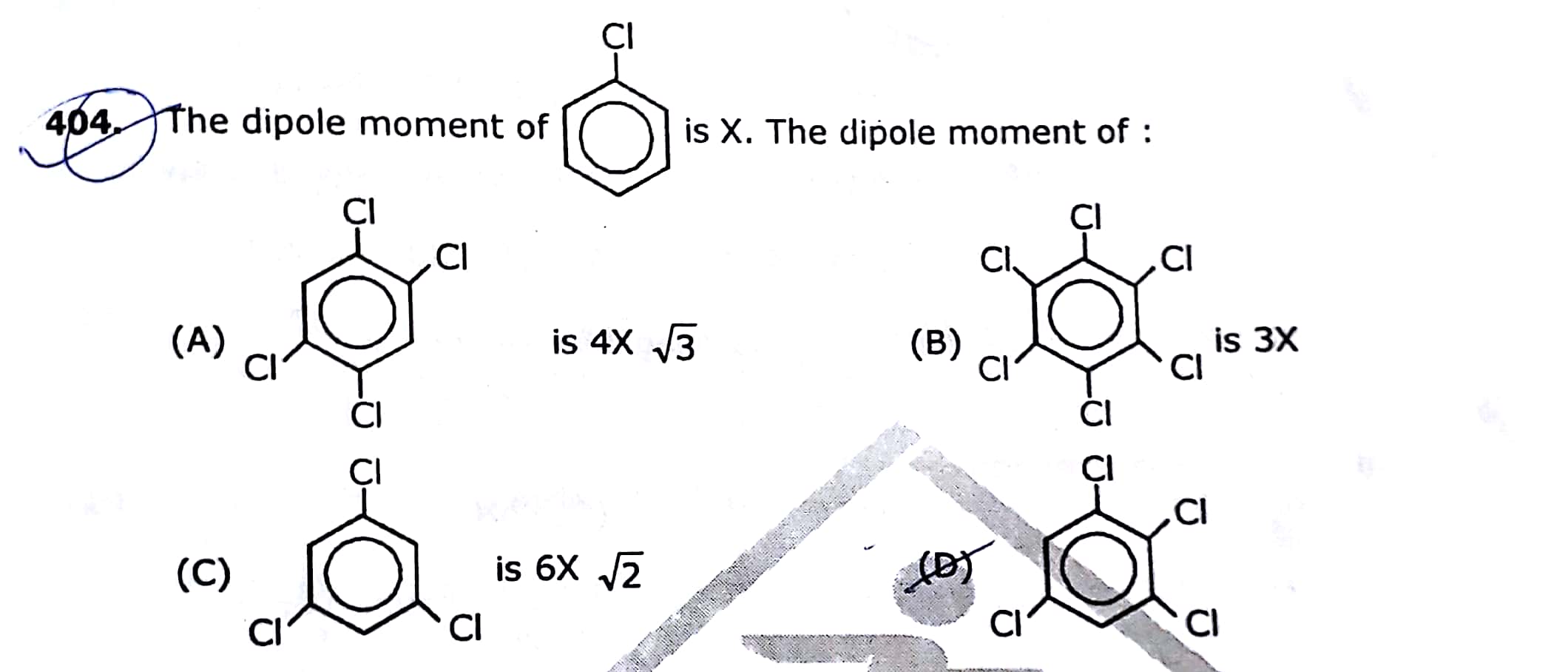
Asked by rsudipto | 24 Dec, 2018, 09:02: AM
The correct answer is option 4
Explanation:
In 1, 2, 3, 5- tetrachlorobenzene, due to two chloro group at position-2 and position-5, the net dipole moment is zero while other two at position 1 and 3 are at an angle of 120°. So, the net dipole moment is equal to X by using the following formula,
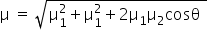
Answered by Ramandeep | 31 Dec, 2018, 02:29: PM
JEE main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE main - Chemistry
Asked by gourishettikrishna | 25 Jan, 2024, 10:01: PM
JEE main - Chemistry
Asked by hemeshsaini2005 | 11 Oct, 2021, 06:13: PM
JEE main - Chemistry
Asked by yasharthshankar | 30 Jun, 2020, 11:13: PM
JEE main - Chemistry
Asked by mdfaraz1182 | 09 Apr, 2020, 11:10: AM
JEE main - Chemistry
Asked by jhajuhi19 | 29 Mar, 2020, 11:19: AM
JEE main - Chemistry
Asked by vidyavikram10 | 29 Mar, 2020, 11:09: AM
JEE main - Chemistry
Asked by rsudipto | 24 Dec, 2018, 09:02: AM