CBSE Class 12-science Answered
sir/ madam,
is it true that if no of bonds in an atom increases then the atom acquires a positive charge on it and when it loses one bond it acquires a negative charge on it ?
why does it happen so?
Asked by kaziryan.05 | 06 Jul, 2021, 11:31: PM
Dear Student,
When number of bond increases it acquires poistive charge it happens in ions like- NH4+ , H3O+
Number of bonds are increased because one extra atom is attached with and one atom from lone pair is released.
For Example in NH3 - One lone pair and 3 bonds are present
In NH4+ ion- One electron from the lone pair is attached one new H and positive charge is due to release of one electon from N.
It is the only posssibility when octet of N is complete with this positve charge.
For negative charge also, To complete the octet of central metal atom one less bond type of structure is formed with negative chargee.
Answered by Ravi | 12 Jul, 2021, 05:06: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 06 Jul, 2021, 11:31: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 01:35: PM
CBSE 12-science - Chemistry
Asked by Sudamkalgunde624 | 31 Dec, 2019, 11:38: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 19 Nov, 2019, 12:39: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 19 Jun, 2019, 09:19: PM
CBSE 12-science - Chemistry
Asked by afiaorpi01 | 22 Mar, 2019, 01:19: AM
CBSE 12-science - Chemistry
Asked by abhitailor158 | 07 Mar, 2019, 04:44: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:44: PM


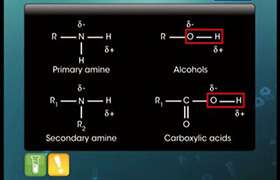

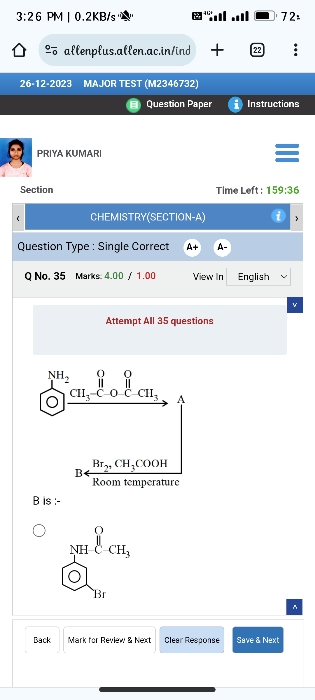
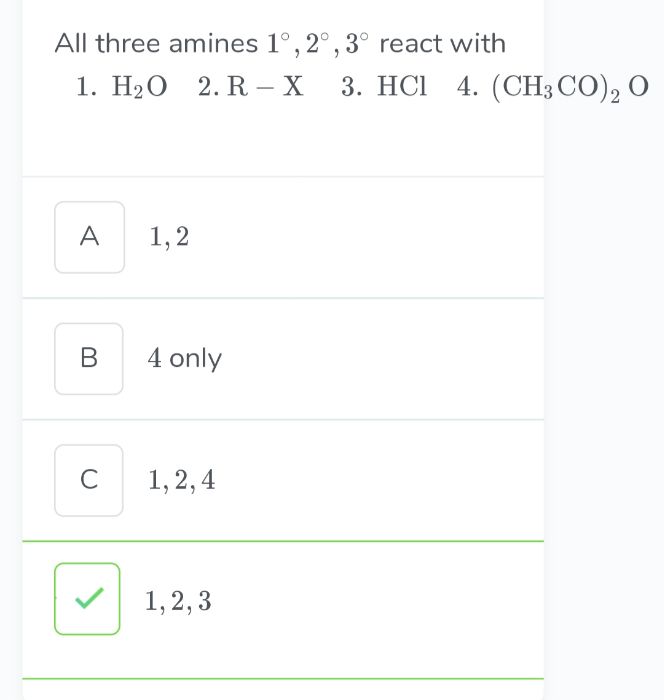
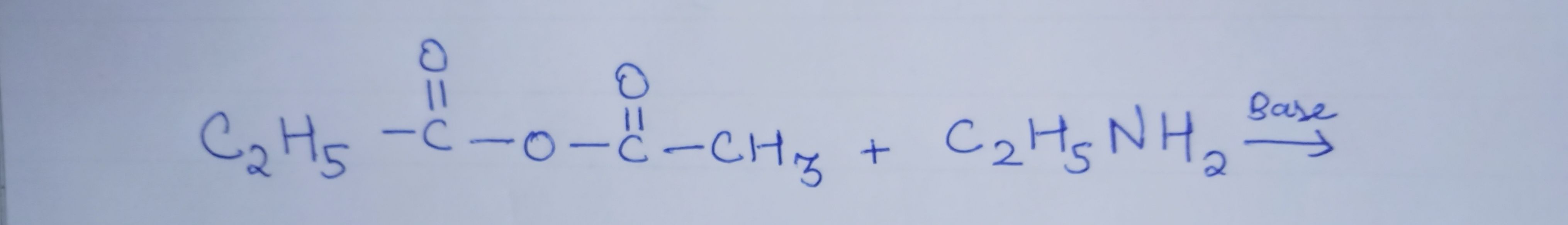
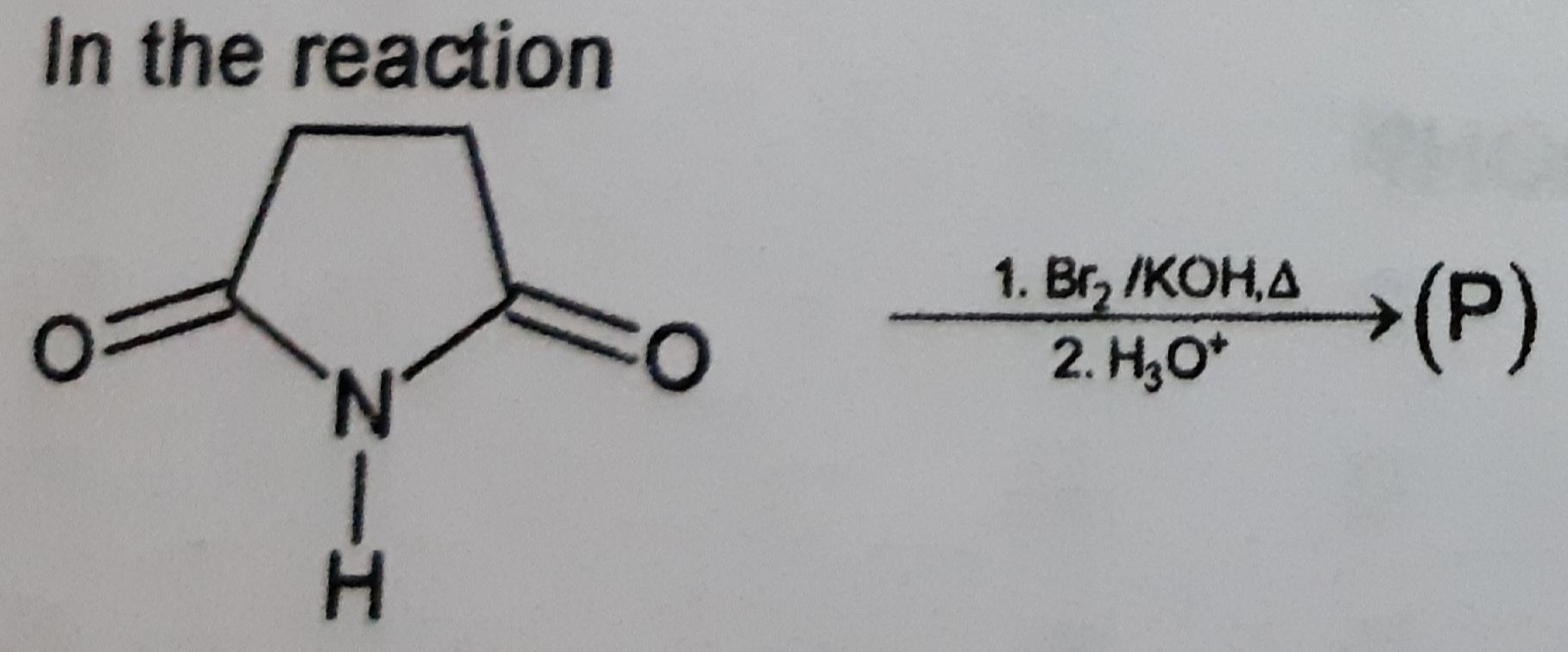

 Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2
Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2