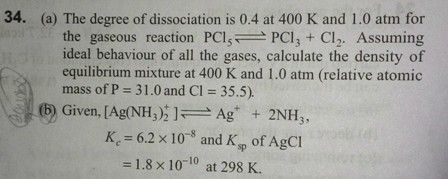NEET Class neet Answered
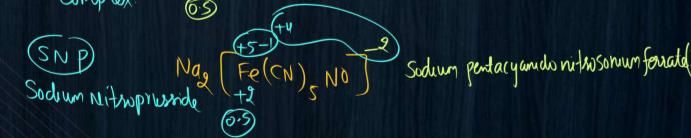
Sir ismein sodium pr charge +2 hai toh box pr -2 aayega aur agr oxidation no iron ka nikalna hai toh NO toh neutral hota aur cyanide pr -1 agr remove Kiya toh iron pr +5 aur box pr -2 total +3 hoga n
Asked by ykrish407 | 20 Sep, 2022, 07:25: PM
Dear Student,
Let x be the oxidation number of Fe.
We know that, the oxidation numbers of Na+,CN− and NO are +1,−1 and 0 respectively.
The sum of the oxidation numbers is zero for whole compound.
Therefore, 2(+1)+x+5(−1)+0=0
x=+3.
Thus, the oxidation state of iron in Na2[Fe(CN)5(NO)] is +3.
Answered by | 21 Sep, 2022, 05:58: PM
NEET neet - Chemistry
Asked by anandibastavade555 | 25 Mar, 2024, 10:01: AM
NEET neet - Chemistry
Asked by ykrish407 | 20 Sep, 2022, 07:25: PM
NEET neet - Chemistry
Asked by priscillaabram | 05 Apr, 2022, 10:02: AM
NEET neet - Chemistry
Asked by singhalakshat6 | 02 Mar, 2022, 07:28: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 06 Jun, 2020, 09:45: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 29 Mar, 2020, 09:39: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 28 Mar, 2020, 10:30: PM
NEET neet - Chemistry
Asked by patra04011965 | 21 Jul, 2019, 11:40: PM