CBSE Class 12-science Answered
Silver crystallizes in face-centred cubic unit cell. Each side of this unit cell has a length of 400 pm. Calculate the radius of the silver atom. (Assume the atoms just touch each other on the diagonal across the face of the unit cell. That is each face atom is touching the four corner atoms.)
Asked by Topperlearning User | 17 Jun, 2016, 09:55: AM
Given, silver crystallizes in fcc unit cell
![]()
Where e is the radius of the silver atom and a is the edge length
Now, edge length = 400 pm = ![]() cm
cm
Thus,
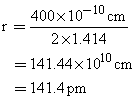
Thus, the radius of the silver atom was found to be 141.4 pm
Answered by | 17 Jun, 2016, 11:55: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by ap996969 | 24 Jan, 2019, 07:08: PM
CBSE 12-science - Chemistry
Asked by shivamsaraswat484 | 23 Mar, 2018, 09:37: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 01 Apr, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:11: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




