CBSE Class 12-science Answered
Show that de Broglie hypothesis of matter waves is in agreement with Bohr's theory.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
According to Bohr's theory, the angular momentum of electron in a stationary orbit is equal to integral multiple of h/![]() i.e.
i.e.
Bohr could give no reason for this quantum condition. However, de Broglie gave the reason for the same. Since electrons move in circular paths, the electron wave must be a circular standing wave that closes on itself .In other words; the circumference of the circular orbit contains a whole number of wavelengths.
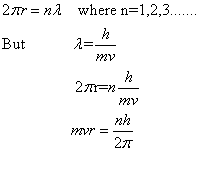
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Physics
Asked by mazhartahsildar143 | 07 May, 2020, 01:44: PM
CBSE 12-science - Physics
Asked by Hemanthrakshitha95 | 03 Mar, 2020, 02:55: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 08 Jan, 2019, 04:33: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:18: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:41: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 19 May, 2014, 04:38: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:49: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




