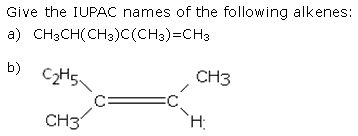
CBSE Class 11-science Answered
Q) plz.. kindly explain the reason behind the answer???

Asked by araima2001 | 28 Mar, 2017, 05:45: AM
The maximum no. of atoms which are oresent in a plane in a molecule of allene is 5.
It can be explained with the help of following structure of allene.

From the above structure, three carbon atoms and two hydrogen atoms present on the right side are present in the same plane and hence shown bonding with simple lines.
The hydrogen atoms present on the left side are shown with one dotted that is below the plane and the other with wedge bonding that is above the plane.
Answered by Prachi Sawant | 29 Mar, 2017, 11:50: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by debupatel8899 | 06 Feb, 2020, 07:04: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:39: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:52: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:55: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:57: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 01 Jun, 2016, 01:50: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 11:20: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 11:28: AM