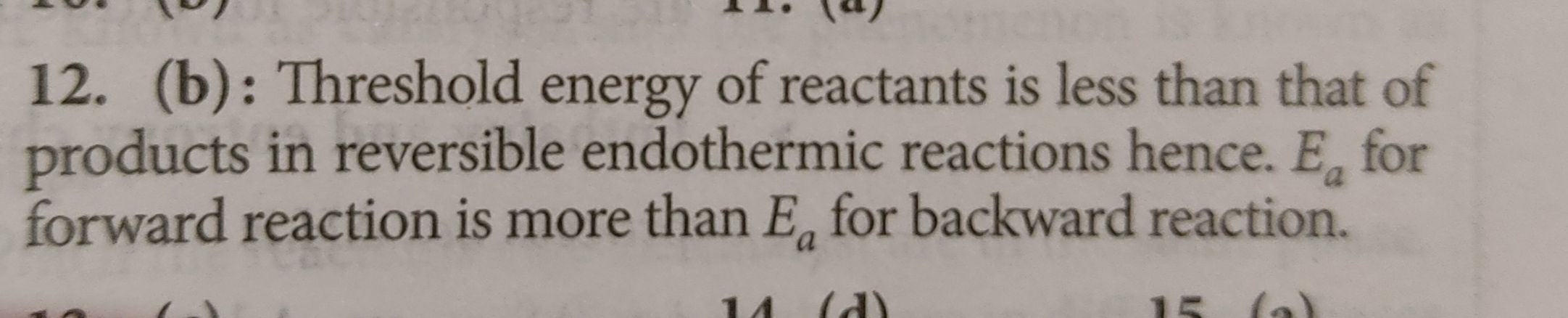NEET Class neet Answered
Q no 106

Asked by manasvijha | 08 Apr, 2019, 09:56: PM
Option (3) is correct.
Given:
Heat of formation of:
CH4 = - 75 kJ/mol
CO2 = -400 kJ/mol
H2O = -240 kJ/mol
The combustion of metahne;
CH4 + 2O2 → CO2 + 2H2O
We know that,
Heat of combustion is;
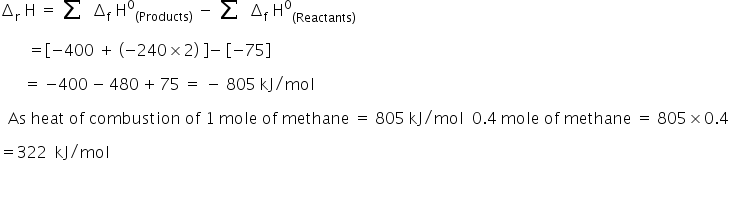
The amount of heat evolved by burning of 0.4 mol of methane is 322 kJ/mol.
Answered by Varsha | 09 Apr, 2019, 11:20: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 10 Aug, 2021, 04:10: AM
NEET neet - Chemistry
Asked by patra04011965 | 12 Nov, 2019, 09:21: AM
NEET neet - Chemistry
Asked by ntg432000 | 06 Mar, 2019, 11:26: AM