JEE Class main Answered
Q) An open bulb containing air at 19 degree Celsius was cooled to a certain temperature at which the number of moles of gaseous molecules increased by 25% what is the final temperature?
Asked by Anish | 08 Jan, 2019, 11:22: PM
Let,
Pressure in bulb = P1
Volume of air = V
Initial temperature = T1 = 19+273
=292 K
Final temperature = T2
Initial moles = n1
Final moles = n2
We have,
n2 = 1.25n1
Using ideal gas equation,
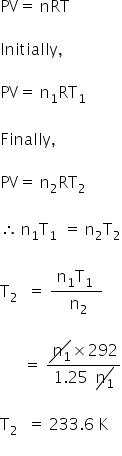
T2 = 233.6 K = 233.6 − 273
= −39.6 °C
The final temperature is −39.6 °C
Answered by Varsha | 09 Jan, 2019, 03:06: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by ruchisharmatbn | 06 Apr, 2024, 08:42: AM
JEE main - Chemistry
Asked by arjuns94037 | 07 Jan, 2024, 12:43: PM
JEE main - Chemistry
Asked by vishakhachandan026 | 08 Aug, 2019, 10:21: AM
JEE main - Chemistry
Asked by ashutosharnold1998 | 08 Aug, 2019, 12:11: AM
JEE main - Chemistry
Asked by vishakhachandan026 | 04 Jul, 2019, 02:59: PM
JEE main - Chemistry
Asked by astutijoshi | 03 Jun, 2019, 05:12: PM
JEE main - Chemistry
Asked by Anish | 14 Mar, 2019, 11:14: AM
JEE main - Chemistry
Asked by inbasri224 | 23 Feb, 2019, 11:48: PM
JEE main - Chemistry
Asked by Anish | 09 Jan, 2019, 11:33: PM
JEE main - Chemistry
Asked by Anish | 08 Jan, 2019, 11:22: PM