CBSE Class 11-science Answered
Q) 1 g mixture of cuprous oxide and cupric oxide was quantitatively reduced to 0.839 g of metallic copper. What was the weight of cupric oxide in the original sample?
(Cu = 63.5, O = 16)
Asked by Anish | 07 Oct, 2018, 12:20: AM
The reaction equations are:
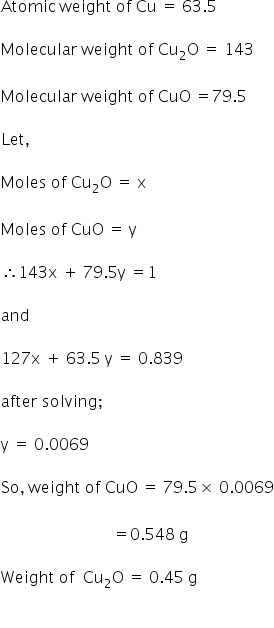
The weight of cupric oxide in the sample is 0.548 g
Answered by Varsha | 10 Oct, 2018, 09:45: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM







