Please solve this question with a detailed explanation.....I am unable to understand the question as well as the solution provided in the book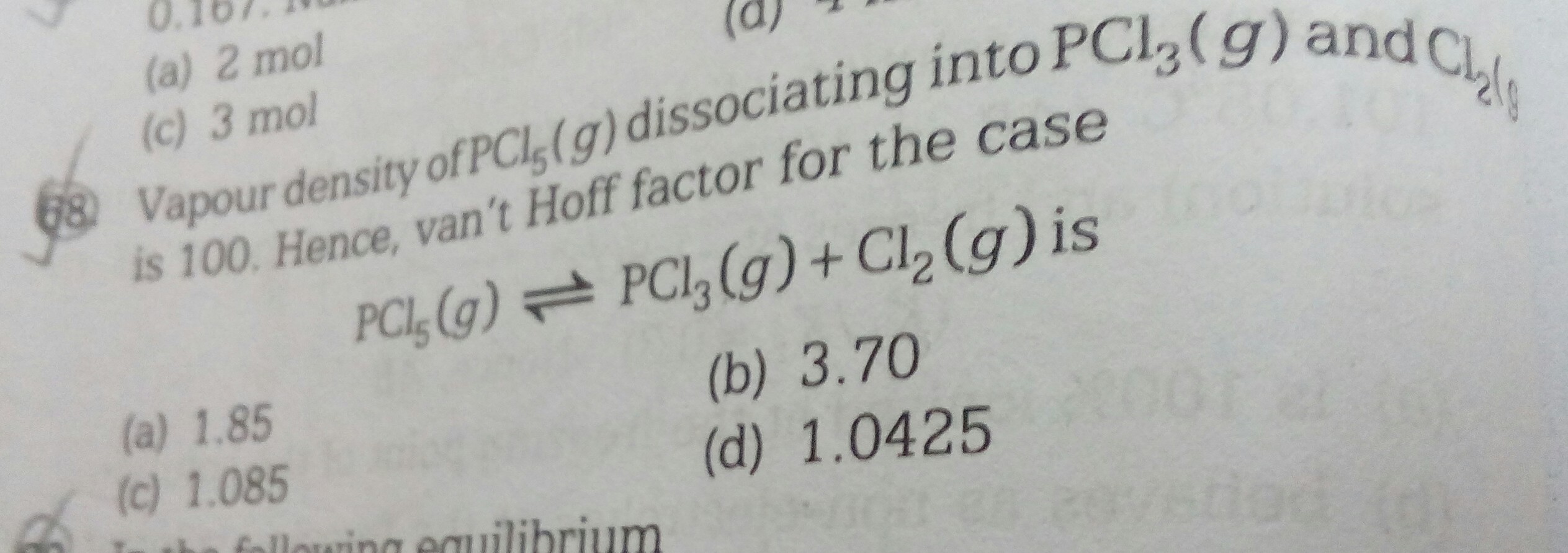
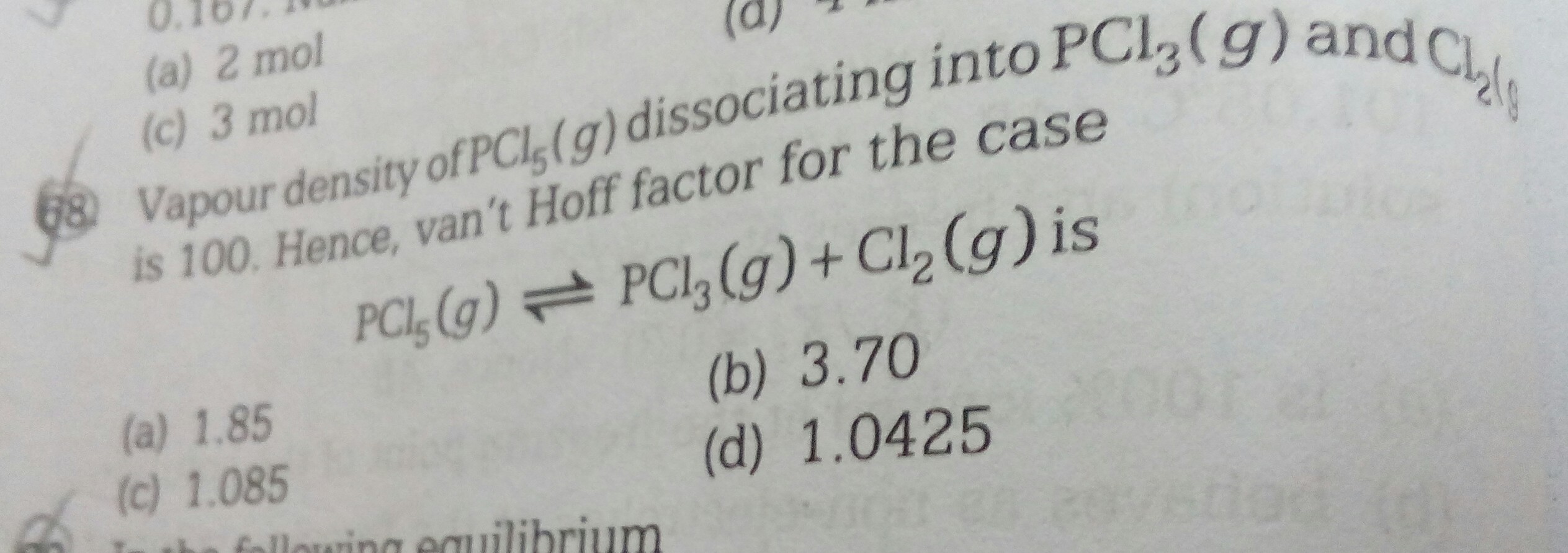
Correct option is (d)
Vapour density = 100
Molecular weight = 2 x VD
So equivalent molecular weight = 208.5/1+ α = 200
208.5 = 1+ α
200
1.04=1+ α
α= 0.04 or 4% is the degree of dissociation.
PCl5 ⇋ PCl3 + Cl2
Mole at equilibrium 1-α α α
Where α is degree of dissociation=0.04
i= 1- α +α + α
i = 1+ α
i =1 + 0.04=1.04
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
