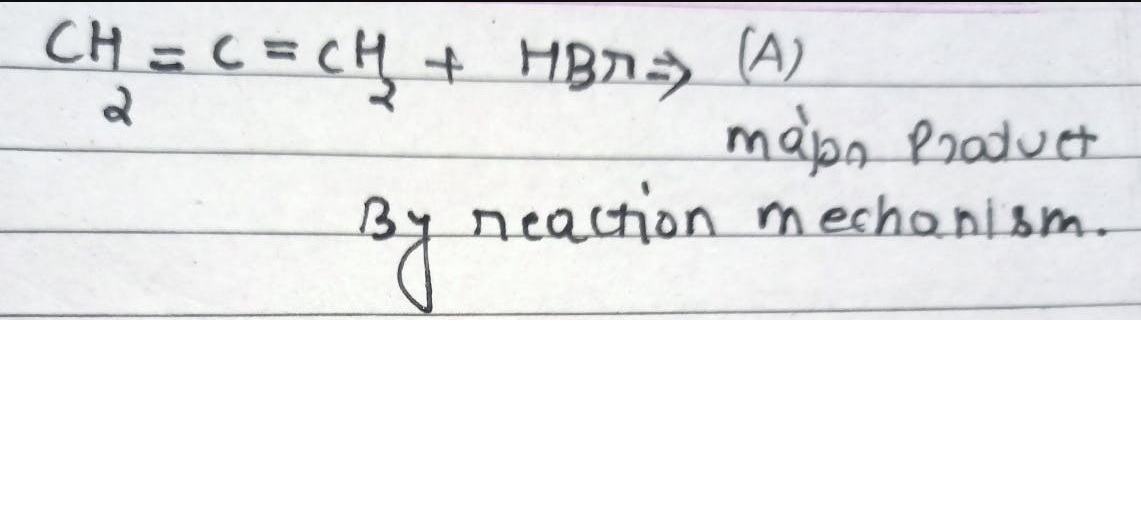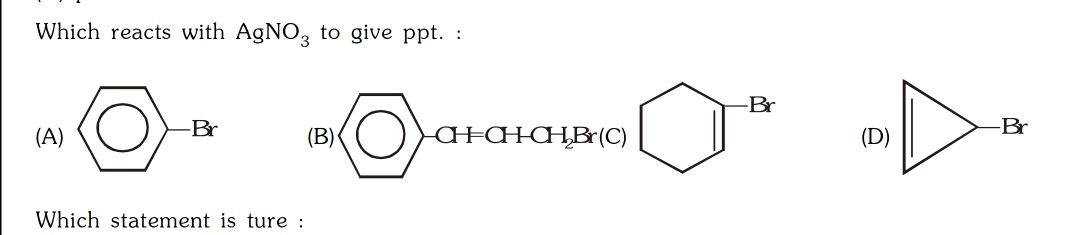CBSE Class 12-science Answered
please explain this why p-methoxybenzyl bromide reacts faster than p-nitrobenzyl bromide with ethanol to form ether?
Asked by vinodjoshi112233 | 29 Sep, 2017, 07:29: AM
Methoxy group is an electron releasing group which stabilises the intermediate carbocation.
Nitro group is an electron withdrawing group which destabilises the intermediate carbocation.
Formation of carbocation is the rate determining step, more the stability of carbocation, faster is the reaction.
Hence, p-methoxybenzyl bromide reacts faster than p-nitrobenzyl bromide with ethanol to form ether.
Answered by Prachi Sawant | 01 Oct, 2017, 01:11: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by surajbhanupatro44 | 07 Nov, 2023, 12:01: AM
CBSE 12-science - Chemistry
Asked by mayamishra9540500880 | 04 Jul, 2022, 07:11: PM
CBSE 12-science - Chemistry
Asked by harshaldpathak | 11 Jun, 2022, 05:37: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 01 Jan, 2021, 09:15: PM
CBSE 12-science - Chemistry
Asked by me.mirzainayat | 14 Nov, 2020, 07:31: AM
CBSE 12-science - Chemistry
Asked by Prachidewangan74 | 02 Oct, 2020, 03:02: PM
CBSE 12-science - Chemistry
Asked by sujithanathan119 | 01 Jun, 2020, 12:00: PM
CBSE 12-science - Chemistry
Asked by ng9045007209 | 21 May, 2020, 07:47: PM
CBSE 12-science - Chemistry
Asked by gangavaramouni | 26 Mar, 2020, 10:33: AM