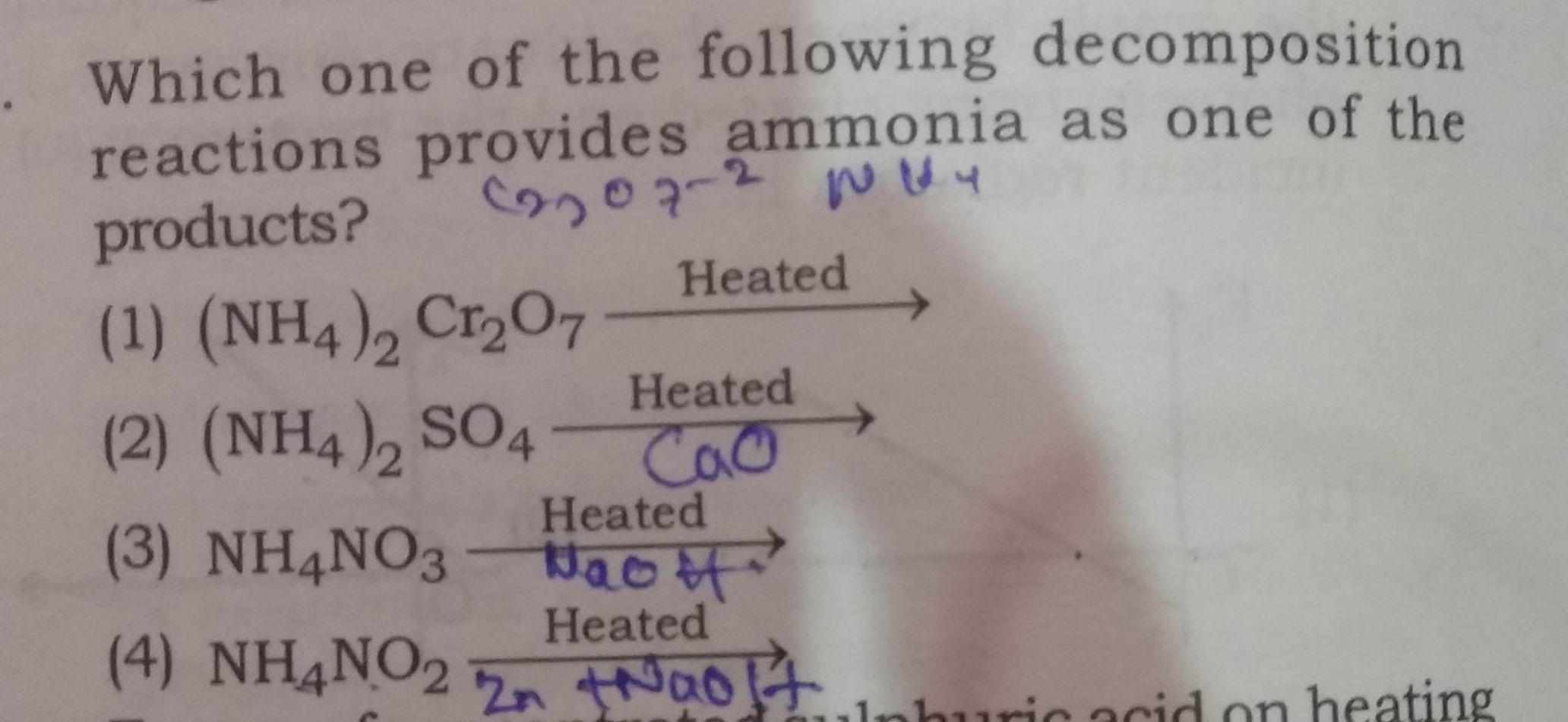
NEET Class neet Answered
Please explain the hybridization of  with structure ?
with structure ?
Asked by Prashant DIGHE | 07 Apr, 2020, 09:53: PM
I3- has sp3d hybridisation and the central I atom has 3 lone pairs and 2 bond pairs. It is a linear molecule.


Answered by Ramandeep | 08 Apr, 2020, 11:08: AM
Concept Videos
NEET neet - Chemistry
Asked by a9460552973 | 02 Mar, 2024, 07:42: AM
NEET neet - Chemistry
Asked by abdulrazz0088 | 02 Jan, 2024, 08:47: PM
NEET neet - Chemistry
Asked by sayesha7862026 | 02 Nov, 2023, 06:32: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 18 Oct, 2020, 04:57: PM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 16 Apr, 2020, 10:11: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 12 Apr, 2020, 03:37: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 07 Apr, 2020, 09:53: PM