CBSE Class 11-science Answered
Please answer
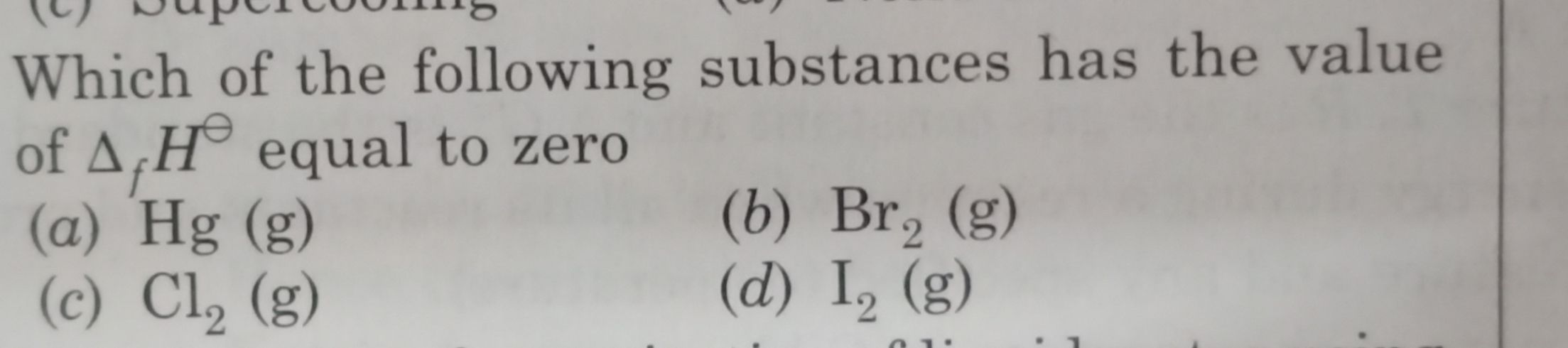
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
Standard enthalpy of formation is zero of the elements in their standard state. Chlorine exists in Cl2(g) form in nature So it is standard form of Chlorine so the standard formation of heat will be zero. In other cases, Hg exists in liquid form, Br2 exists in liquid and I2 exists in solid form in nature, so the standard enthalpy of formation cannot be zero for other given elements.
Answered by Ravi | 27 Sep, 2019, 07:34: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 07:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 03:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 05:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 04:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 05:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:24: AM