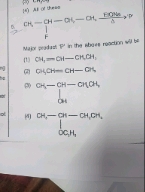
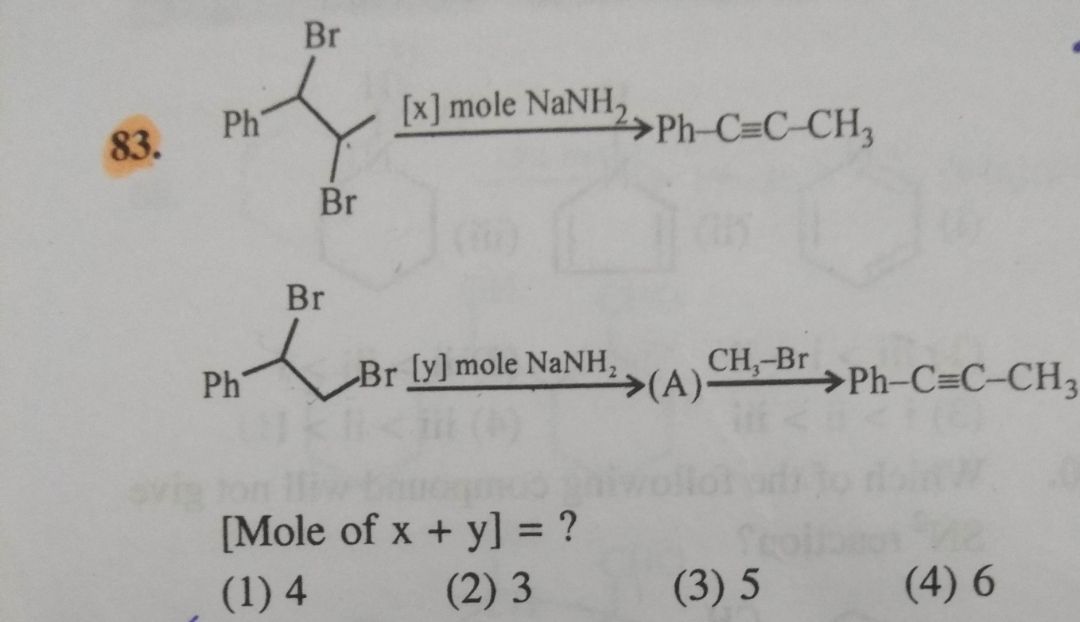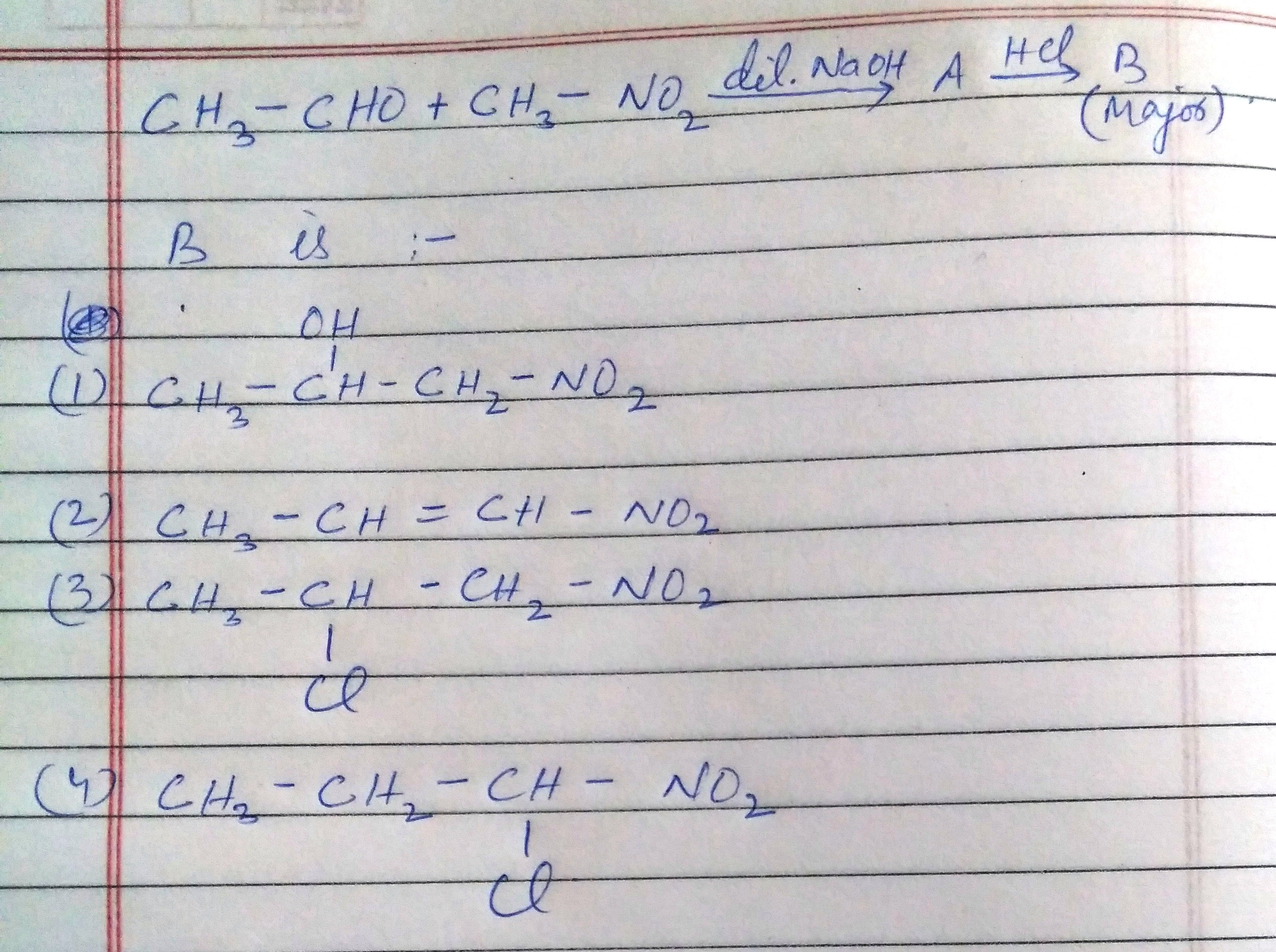NEET Class neet Answered
please answer this
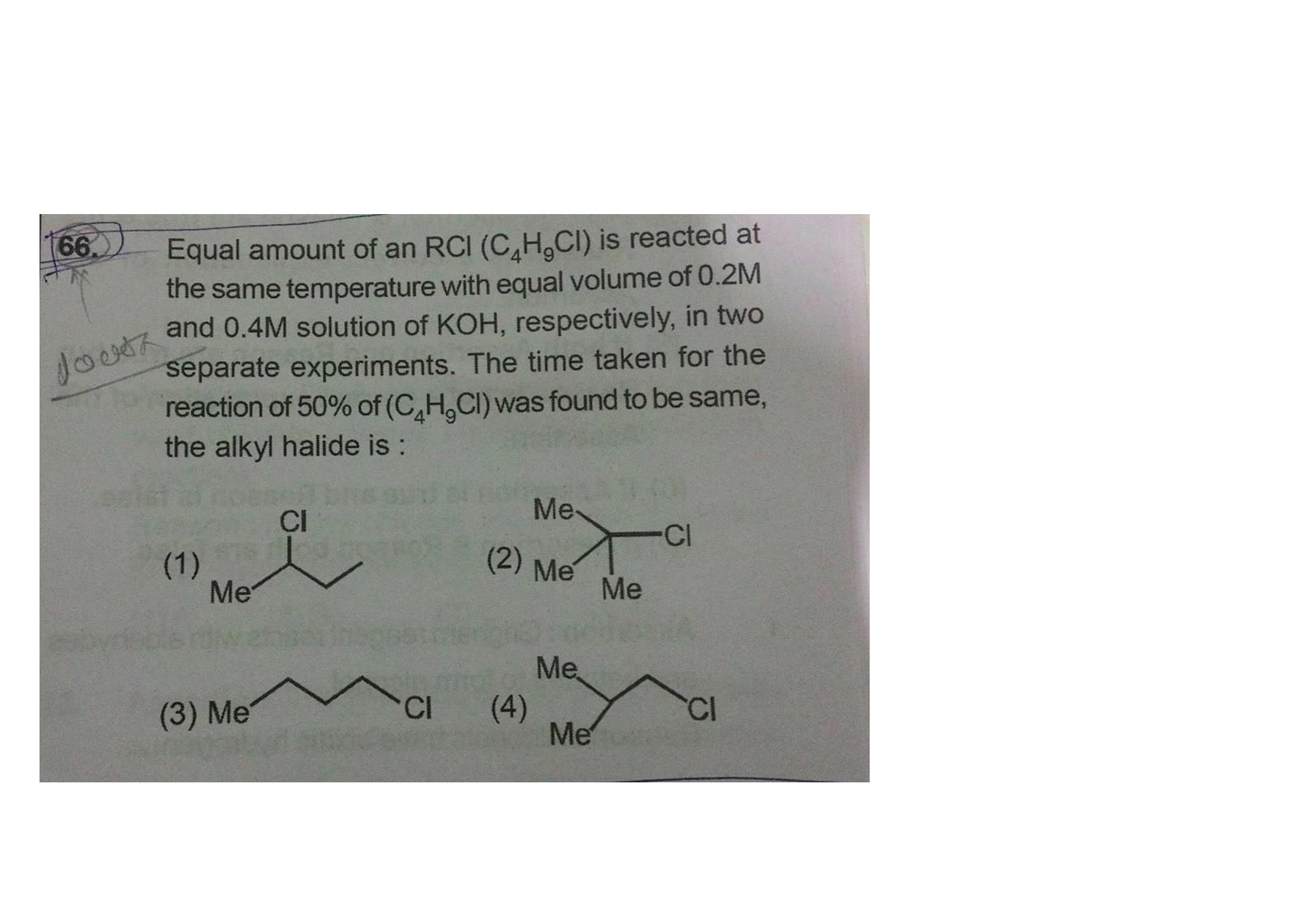
Asked by Prashant DIGHE | 19 Dec, 2019, 10:44: PM
In the above experiment, the alkyl halide acts as a substrate and HO- from KOH as a nucleophile hence this is a nucleophilic substitution reaction.
As the reaction proceeds we get the same amount of product which suggests that the rate of reaction does not depend on the amount of nucleophile.
From this information, we can conclude that this is a unimolecular nucleophilic substitution reaction. Hence the substrate will be a compound from option 2 which can form a stable tertiary carbocation.
Hence option 2 is the correct answer.
Answered by Ramandeep | 20 Dec, 2019, 05:21: PM
NEET neet - Chemistry
Asked by hk62929363 | 04 Mar, 2024, 03:56: AM
NEET neet - Chemistry
Asked by samramojuru | 22 Feb, 2024, 04:18: PM
NEET neet - Chemistry
Asked by swetadayal036 | 15 Jan, 2024, 08:04: PM
NEET neet - Chemistry
Asked by sabhachoudhary0786 | 15 Sep, 2023, 04:53: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 05 Sep, 2020, 05:35: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 11 Aug, 2020, 04:25: AM
NEET neet - Chemistry
Asked by subhasanth | 29 Apr, 2020, 10:53: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 13 Apr, 2020, 08:27: AM
NEET neet - Chemistry
Asked by harunabbas | 08 Apr, 2020, 02:13: PM
NEET neet - Chemistry
Asked by prakriti12oct | 30 Mar, 2020, 01:23: AM