NEET Class neet Answered
Please answer this
Asked by Prashant DIGHE | 08 Oct, 2019, 09:42: PM
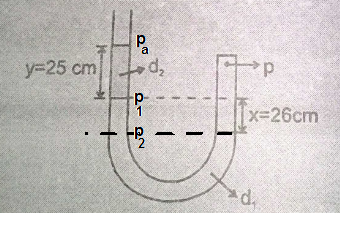
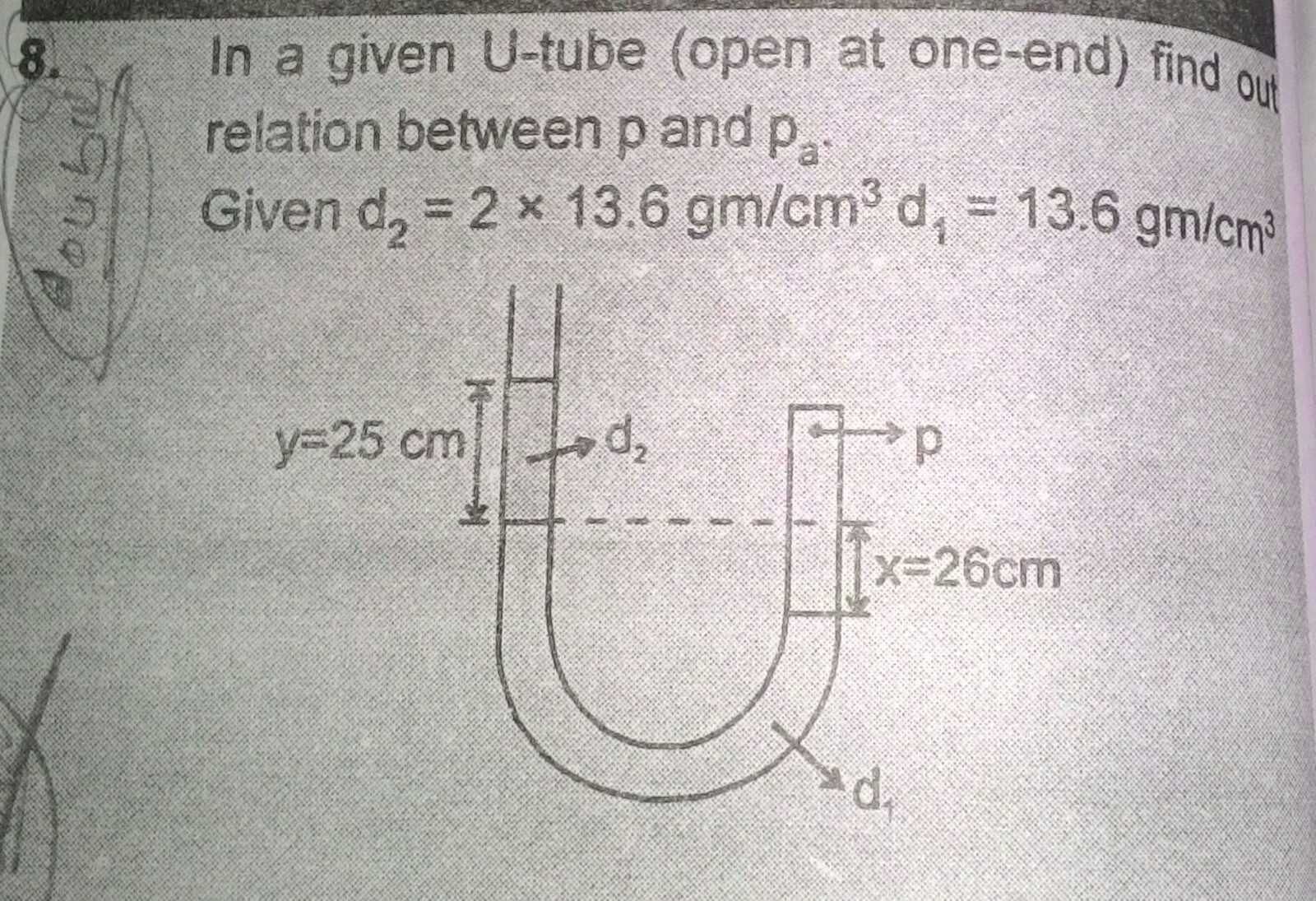
Let Pa is atmospheric pressure
density of liquid d2 is twice of mercury density 13.6 g/cm3
Pressure P1 = Pa + 2×(25 cm of mercury pressure)
density of liquid d1 is mercury density 13.6 g/cm3
Pressure P2 = P1 + 26 cm of mercury pressure = Pa + 76 cm of mercury pressure
since atmospheric pressure Pa is 76 cm of mercury pressure, we get pressure at closed limb of u-tube, p = 2 Pa
Answered by Thiyagarajan K | 09 Oct, 2019, 10:16: AM
Application Videos
NEET neet - Physics
Asked by shatakshibhatt9 | 20 Apr, 2024, 07:52: PM
NEET neet - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM
NEET neet - Physics
Asked by gouranshi84 | 17 Apr, 2024, 05:23: PM
NEET neet - Physics
Asked by sojusvi | 17 Apr, 2024, 01:12: PM