NEET Class neet Answered
Please answer the following question with explanation

Asked by deepakudgiri29 | 25 Dec, 2018, 05:19: PM
The reaction is as;
(NH4)2 SO4 + 2NaOH → 2NH3 + Na2SO4 + 2H2O
2NaOH + H2SO4 → Na2SO4 + 2H2O
5 ml 0.2 N H2SO4 solution used for titration,
So,
0.2 N H2SO4 = 0.1 M H2SO4
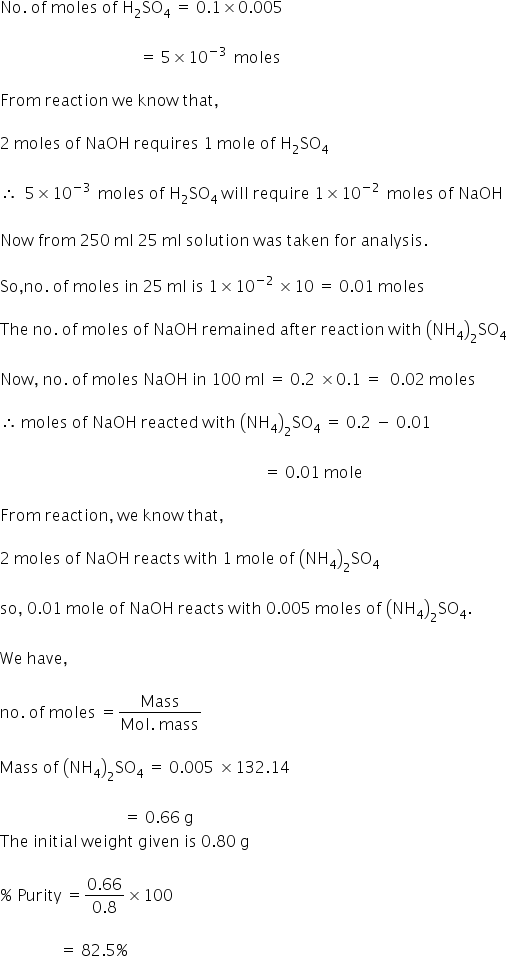
The percentage purity of the (NH4)2 SO4 samples is 82.5%.
Answered by Varsha | 26 Dec, 2018, 04:37: PM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
for a gaseous reaction at 300k, ûÂôôH-ûÂôôU=-4.98kj assuming that r=8.3JK^-1mol^-1 ûÂôôn(g) is


Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM



















