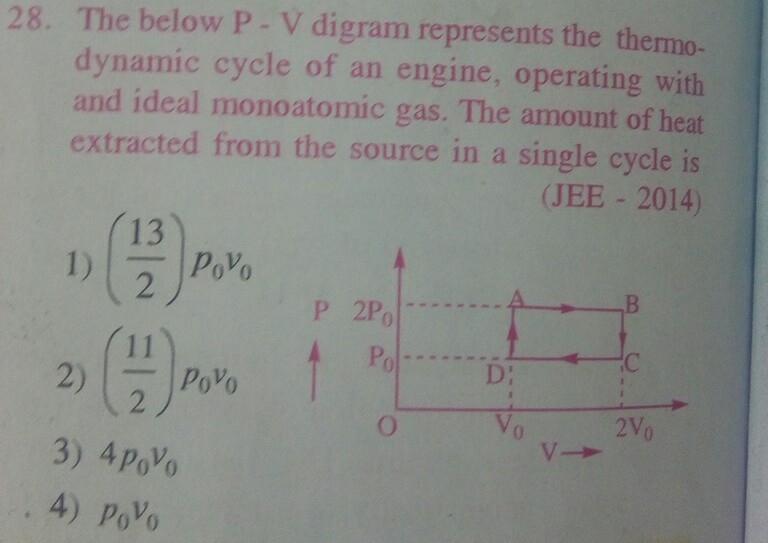
For monoatomic ideal gas, Heat absorbed/released at constant volume process = n×CV×ΔT = n×(3/2)×R×ΔT..................(1)
where n is number of moles, CV is specific heat at constant volume, R is gas constant and ΔT is increase/decreas of temperature
For monoatomic ideal gas, Heat absorbed/released at constant pressure process = n×CP×ΔT = n×(5/2)×R×ΔT..................(2)
Let us start from point D in the given P-V diagram.
Let us assume when the gas is at the state represented by point D in the P-V diagram, its temperature is T0 .
When the gas pressure increases from P0 to 2P0 at constant volume V0 during the process represented by DA in the P-V diagram,
its temperaure is increased from T0 to 2T0 .
Hence heat absorbed in the DA part of cycle is, QDA = (3/2)×n×R×T0 ................................................(3)
For the AB part of cycle, Volume doubles at constant pressure. Hence temperature at B is 4T0 .
Heat aborbed in the AB part of cycle, QAB = (5/2)×n×R×2T0 = 5×n×R×T0 .................................................(4)
For the BC part of cycle, Pressure is decreased to half. Hence temperature at C is 2T0 .
Heat released in the BC part of cycle, QBC = -(3/2)×n×R×2T0 = -3×n×R×T0 .................................................(5)
Heat released in the CD part of cycle, QCD = -(5/2)×n×R×T0 .................................................(6)
net Amount of heat absorbed in full cycle = QDA + QAB + QBC + QCD = [ (3/2) + 5 - 3 - (5/2) ]×n×R×T0 = n×R×T0 = P0×V0
------------------------------------------------------------------------------------------------
Answer to this problem can be obtained in alternate way.
For a complete cycle, at a given point in P-V diagram that represents the state of the gas, change in internal energy ΔE is zero.
Hence ΔE = ΔQ+ΔW =0 ..............................(7)
we have ΔW = workdone in the path AB + workdone in the path CD = -(2P0×V0 ) + ( P0×V0) = - ( P0×V0) ...................(8)
from eqn.(7) and (8), we have ΔQ = - ΔW = P0×V0