NEET Class neet Answered
Please answer the following question with explanation
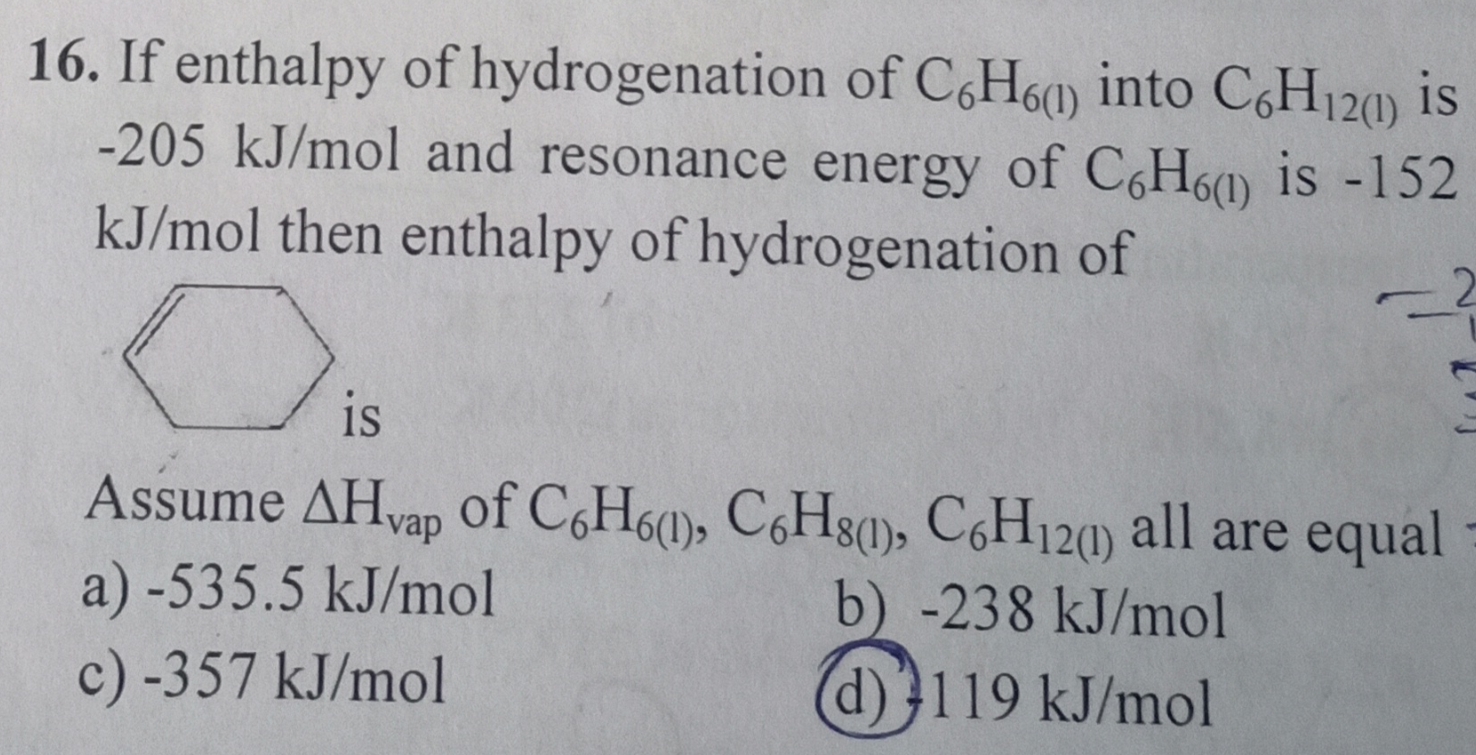
Asked by deepakudgiri29 | 25 Dec, 2018, 05:14: PM
C6H6(l) + 3H2 → C6H12(l) ΔHHydrogenation = -205KJ/mol
Total Energy = Resonance Energy + ΔHHydrogenation
= -(205 + 152)/3 KJ/Mol = -119 KJ/Mol (option d) (Total Energy = Average of the energy required for Benzene )
Answered by Sumit Chakrapani | 26 Dec, 2018, 09:38: AM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM
NEET neet - Chemistry
Asked by ghousiakaneez | 03 Apr, 2024, 12:55: PM




















