NEET Class neet Answered
Please answer the following question with explanation
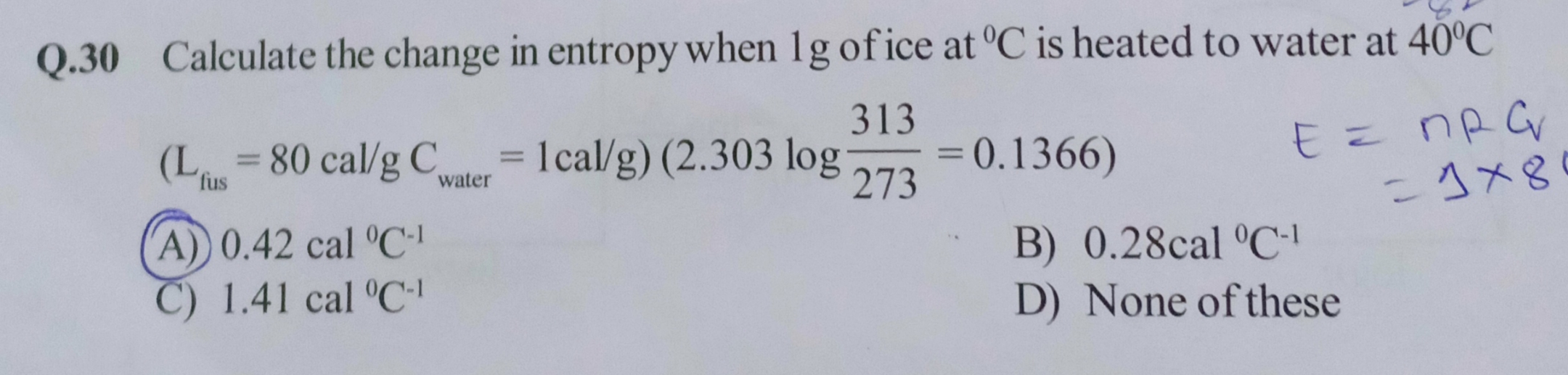
Asked by deepakudgiri29 | 02 May, 2019, 11:08: PM
Change in entropy ΔS = entropy change for ice to melt + entropy change when heated from 0°C to 40°C
= (ΔQ/Ti) + m×Cp×ln( Tf / Ti)
ΔQ = latent heat of fusion of ice = 80 cal/g
Ti = ice temperature = 273 K , Tf = final temperature = 313 K
m = mass of ice = 1 g
CP = Specific heat of ice = 1 cal/( g °C )
ΔS = (80/273) + 1×1×0.1366 ≈ 0.42 cal/°C
Answered by Thiyagarajan K | 03 May, 2019, 02:22: PM
Application Videos
NEET neet - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM
NEET neet - Physics
Asked by gouranshi84 | 17 Apr, 2024, 05:23: PM
NEET neet - Physics
Asked by sojusvi | 17 Apr, 2024, 01:12: PM