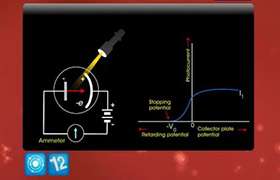
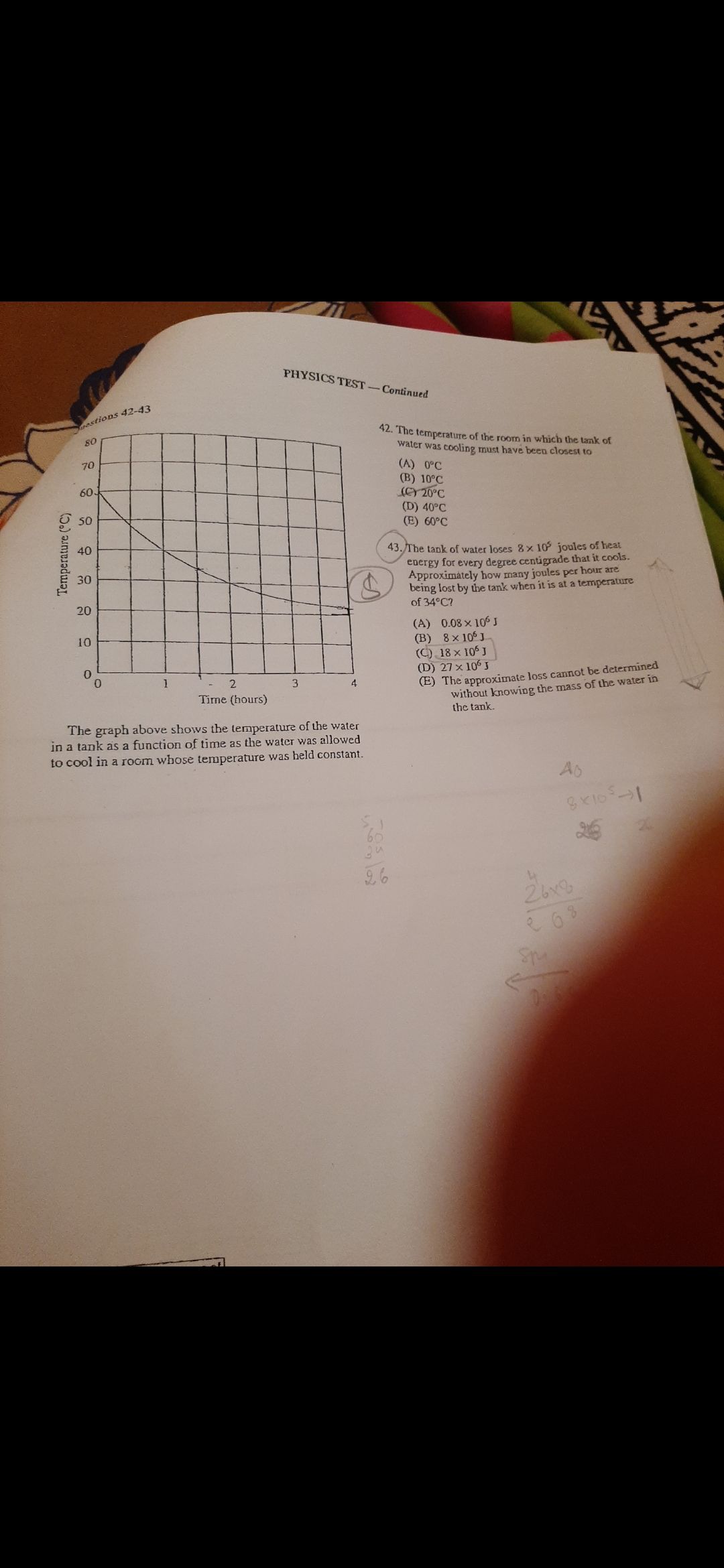
CBSE Class 12-science Answered
The intermolecular distances in a liquid are greater than those in a solid. Similarly, the intermolecular distances in the gaseous state are greater than those in a liquid. Therefore during the change from solid to liquid state or from liquid to gaseous state, work must be done against the intermolecular forces to increase the intermolecular distances. Also the change from solid to liquid state or from liquid to gaseous state is usually accompanied with an increase in volume of the substance. During such expansion, the substance has to perform external work.
Thus the latent heat (L) absorbed by the substance during the change of state is utilised in the following two ways.
(i) Part of the latent heat is used to perform internal work against the intermolecular forces of attraction, in order to increase the separation between the molecules. This part is called the internal latent heat (L)
(ii) Remaining part of the latent heat is used to perform external work during expansion. This part is called the external latent heat (Le).
The latent heat L is thus the sum of the internal and external latent heats.
Hope this helps.
Thanking you
Team
Topperelarning.com