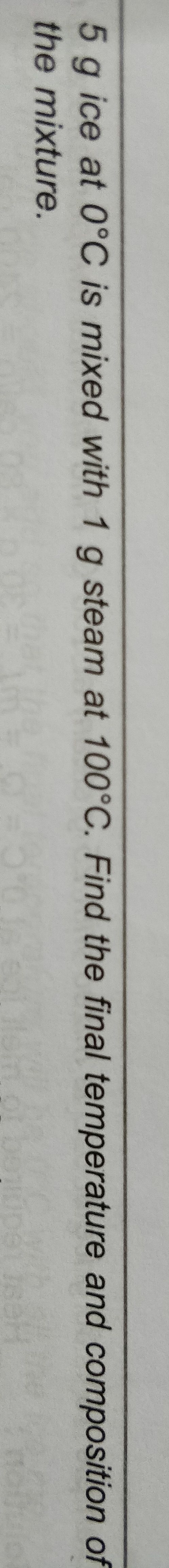NEET Class neet Answered
physics
Asked by rc1927633 | 31 May, 2022, 11:41: AM
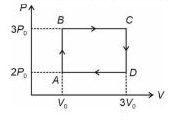
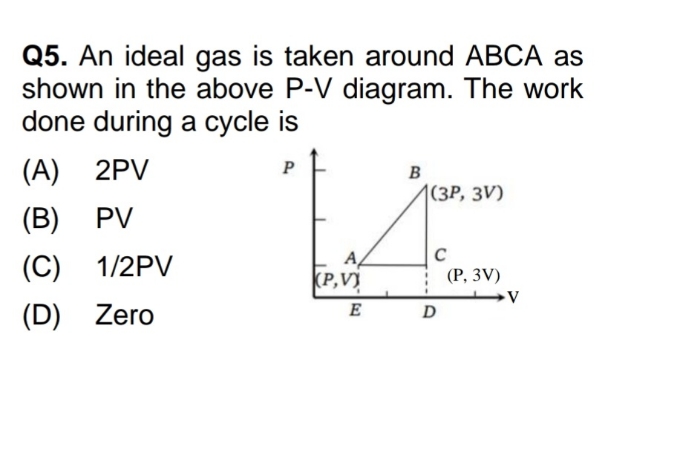
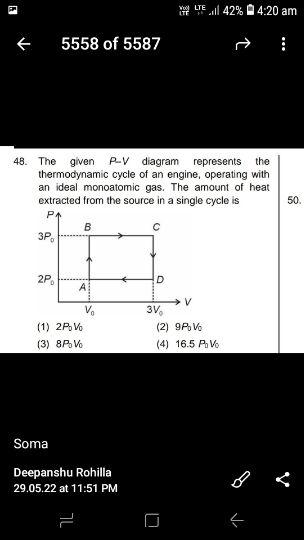
Process AB is constant volume heating process. In this path , TB > TA .
( T is temperature , subscript indicates the state )
Process BC is constant pressure heating process. In this path , TC > TB .
Process CD is constant volume cooling process. In this path , TD < TC .
Process DA is constant pressure cooling process. In this path , TA < TD .
Hence heat is extracted from source only in the paths AB and BC .
Heat extracted in path AB , QAB = n Cv ( TB - TA ) .............................(1)
where n is number of moles of monoatomic gas used in this cycle and
Cv = (3/2)R is constant volume specific heat, R is universal gas constant
Hence we write eqn.(1) as
QAB = n (3/2) R [ TB - TA ] = ( 3/2 ) [ n R TB - n R TA ]
QAB = ( 3/2 ) [ ( 3 Po ) Vo - (2Po ) Vo ] = ( 3/2 ) Po Vo
Heat extracted in path BC , QBC = n Cp ( TC - TB ) .............................(2)
where Cp = (5/2)R is constant pressure specific heat
Hence we write eqn.(2) as
QBC = n (5/2) R [ TC - TB ] = ( 5/2 ) [ n R TC - n R TB ]
QBC = ( 5/2 ) [ ( 3 Po ) 3Vo - (3Po ) Vo ] = 15 Po Vo
Net Heat extracted in the cycle = QAB + QBC = [ (3/2 + 15 ] Po Vo = 16.5 Po Vo
Answered by Thiyagarajan K | 31 May, 2022, 12:55: PM
NEET neet - Physics
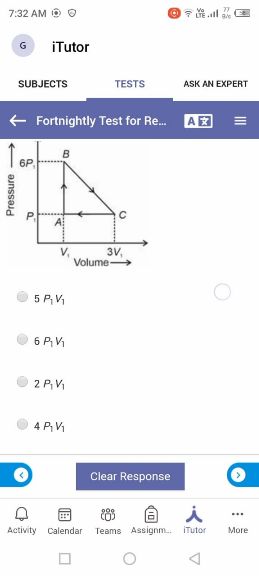
Asked by yadavaradhana9335 | 19 Feb, 2024, 04:53: PM
NEET neet - Physics
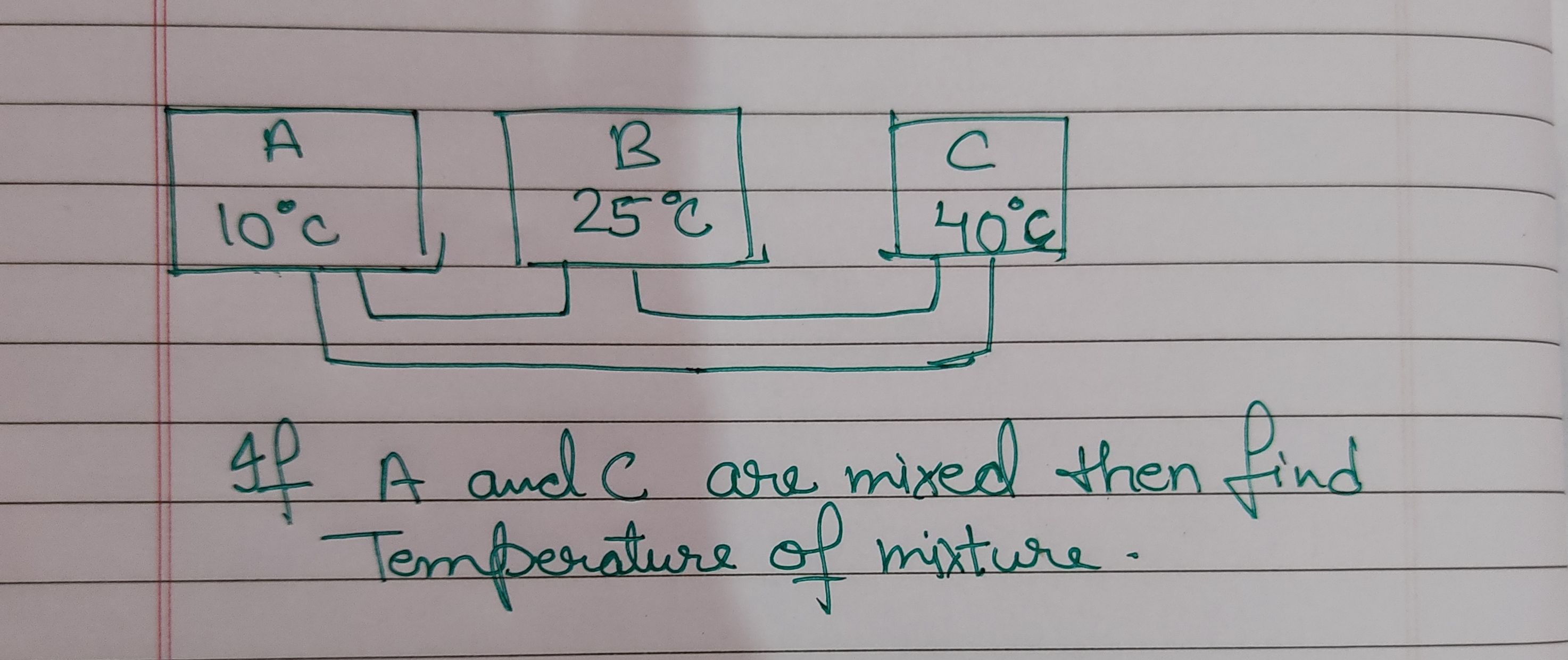
Asked by sujitjana971 | 18 Dec, 2022, 05:23: PM
NEET neet - Physics
Asked by takshitashu46 | 09 Feb, 2022, 06:01: PM
NEET neet - Physics
Asked by jhajuhi19 | 18 Aug, 2021, 03:21: AM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 05:23: PM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 05:23: PM