NEET Class neet Answered
Objective type of question

Asked by shahlaghazal009 | 24 Apr, 2019, 11:28: AM
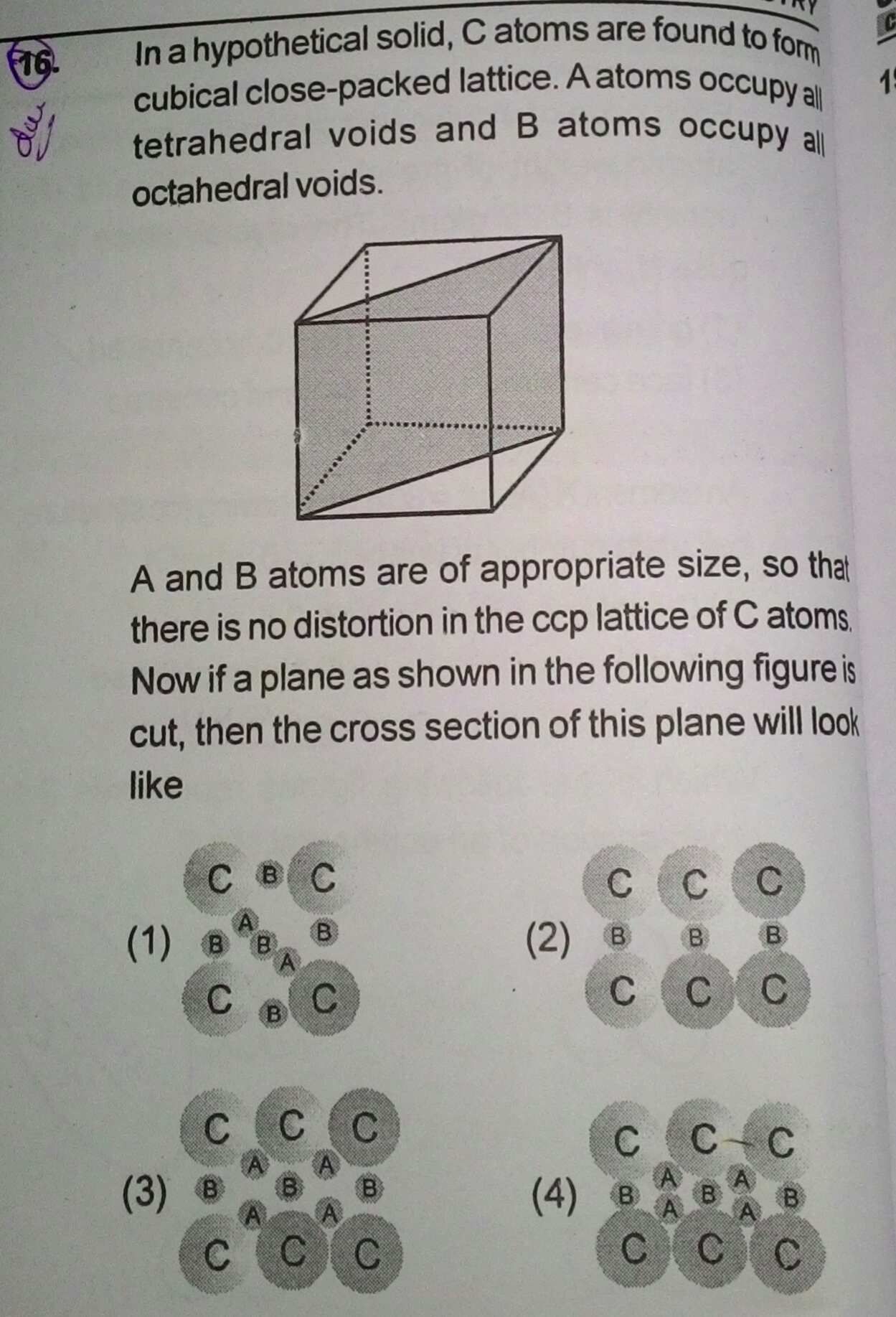
Question 37:
The density of argon (fcc) is 1.83 g/cm3 at 20 ºC. What is the length of an edge of a unit cell?
Given:
Density ρ = 1.83 g/cm3
Molar mass = 40
NA = 6.022 × 1023
We know,
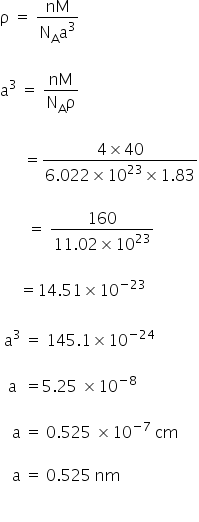
Edge length is 0.525 nm.
Answered by Varsha | 25 Apr, 2019, 07:49: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 30 Jul, 2019, 07:59: AM
NEET neet - Chemistry
Asked by shahlaghazal009 | 24 Apr, 2019, 11:28: AM
NEET neet - Chemistry
Asked by inbasri224 | 23 Feb, 2019, 11:46: PM