CBSE Class 11-science Answered
Name the type of forces present in CH3Cl and PCl3 molecule.
Asked by Topperlearning User | 17 Jun, 2016, 11:30: AM
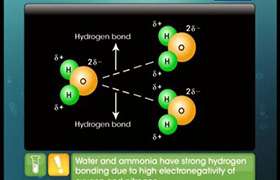
CH3Cl and PCl3 molecules have permanent net dipoles so they have dipole-dipole interactions.
Answered by | 17 Jun, 2016, 01:30: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by neerudhawanbpsmv | 28 Jun, 2021, 09:22: AM
CBSE 11-science - Chemistry
Asked by swatipuspapatel | 10 Jun, 2020, 06:19: PM
CBSE 11-science - Chemistry
Asked by vanyashree106 | 13 Dec, 2019, 09:38: PM
CBSE 11-science - Chemistry
Asked by Asif.k1992 | 12 Sep, 2019, 02:41: PM
CBSE 11-science - Chemistry
Asked by ajitbhot73 | 25 Aug, 2019, 11:52: AM
CBSE 11-science - Chemistry
Asked by design1.bharatifire | 21 Jun, 2019, 06:35: PM
CBSE 11-science - Chemistry
Asked by anitabahuguna | 04 May, 2019, 09:57: PM
CBSE 11-science - Chemistry
Asked by Jatinaa786 | 19 Sep, 2018, 02:53: AM
CBSE 11-science - Chemistry
Asked by arjunshrivastav668 | 17 Mar, 2018, 10:21: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM