CBSE Class 12-science Answered
my question is you say in 3 part in electrichemical cell that cu+/cu will be the greater electrod potential soo those have larger electrod potential so we prefer them cathod and those are lesser value those are anode little confusion
see in part 3 timming 00:05:45
and why we use electrod potential what is the use
Asked by saggugurupal | 15 Jun, 2016, 12:21: PM
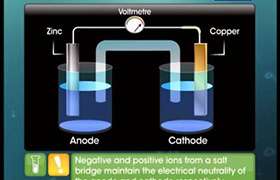
Cathode: The electrode at which reduction takes place with respect to standard hydrogen electrode has reduction potential which is given a positive sign.
Anode: The electrode at which oxidation takes place with respect to standard hydrogen electrode has a positive oxidation potential or expressed as reduction potential, it will have a negative sign.
Thus, in the electrochemical series,
The metals which have positive or larger reduction potential, can behave as good oxidising agent. Hence they act as cathode.
The metals which have negative reduction potential, can behave as good reducing agent. Hence they can act as anode.
Answered by Prachi Sawant | 17 Jun, 2016, 11:44: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 08:39: PM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 08:51: PM
CBSE 12-science - Chemistry
Asked by Poojanisha1988 | 19 Jul, 2023, 09:59: PM
CBSE 12-science - Chemistry
Asked by jajimuji2306 | 03 Apr, 2022, 01:38: PM
CBSE 12-science - Chemistry
Asked by Harshfarwaha | 23 Jul, 2020, 03:27: PM
CBSE 12-science - Chemistry
Asked by sourabhkumar9923 | 19 May, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by ssharondaniel | 27 Jul, 2019, 06:22: PM
CBSE 12-science - Chemistry
Asked by kripanjalihimansu | 28 Feb, 2019, 06:57: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 12 Sep, 2018, 05:40: PM