CBSE Class 12-science Answered
Monochromatic radiation of frequency greater than threshold value is incident on a metal. Why do all the photoelectrons not come with the same kinetic energy?
Asked by Topperlearning User | 19 May, 2014, 02:52: PM
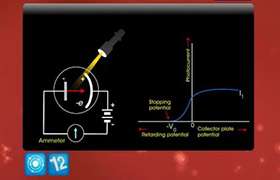
Work function of the metal is the minimum energy required just to get an electron out of its surface. Since electrons are present in a continuous band of levels, different electrons require different amounts of energy to get out of the atom.
Electrons which are least tightly held that is just at the surface of the metal, comes from the metal with maximum kinetic energy. However electrons below the surface are involved in collisions on their way out of the surface and, therefore emerge with energy which is less than the maximum value.
Hence, photoelectrons knocked off by a monochromatic radiation posses different energies.
Answered by | 19 May, 2014, 04:52: PM
Concept Videos
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 07 Apr, 2024, 11:23: AM
CBSE 12-science - Physics
Asked by shivakumarshreyas24 | 01 Mar, 2020, 08:12: AM
CBSE 12-science - Physics
Asked by khushimassey437 | 31 May, 2019, 08:41: AM
CBSE 12-science - Physics
Asked by manasvijha | 19 Mar, 2019, 07:17: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM