CBSE Class 9 Answered
Mole concept
Asked by mishraadarsh865 | 06 Mar, 2019, 01:31: PM
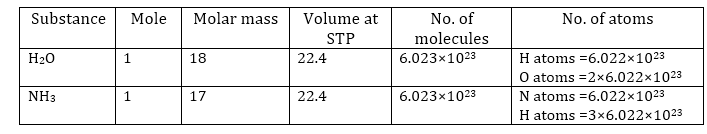
1 mole of a substance is equal to its atomic mass or molecular mass expressed in grams.
The atomic mass of sodium is 23 grams.
Therefore, 23 grams of sodium is equal to one mole of sodium atoms.
Similarly, the molecular mass of oxygen (O2) = 2 × Atomic mass of oxygen
= 2 × 16 = 32 g
So, 32 grams of oxygen is equal to one mole of oxygen molecules.
1 mole (of anything) = 6.022 × 1023 in number
Answered by Varsha | 06 Mar, 2019, 02:34: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by gauravsingh36428 | 14 Mar, 2022, 07:44: PM
CBSE 9 - Chemistry
Asked by krishnaahlawat062 | 27 May, 2021, 09:01: PM
CBSE 9 - Chemistry
Asked by kinshukgoel | 12 Sep, 2020, 01:26: PM
CBSE 9 - Chemistry
Asked by saraswatiupadhaya111 | 26 Aug, 2020, 08:54: AM
CBSE 9 - Chemistry
Asked by sonydatta5 | 24 Aug, 2020, 10:18: AM
CBSE 9 - Chemistry
Asked by Minajoshi62 | 02 Feb, 2020, 09:01: PM
CBSE 9 - Chemistry
Asked by tarunvignesh2005 | 12 Jan, 2020, 05:46: PM
CBSE 9 - Chemistry
Asked by Virenpareek3 | 23 Nov, 2019, 04:13: PM
CBSE 9 - Chemistry
Asked by fathimafero | 11 Sep, 2019, 02:00: PM
CBSE 9 - Chemistry
Asked by abhishekverma0300 | 27 Aug, 2019, 12:41: AM