Mark as true or false and hence justify.......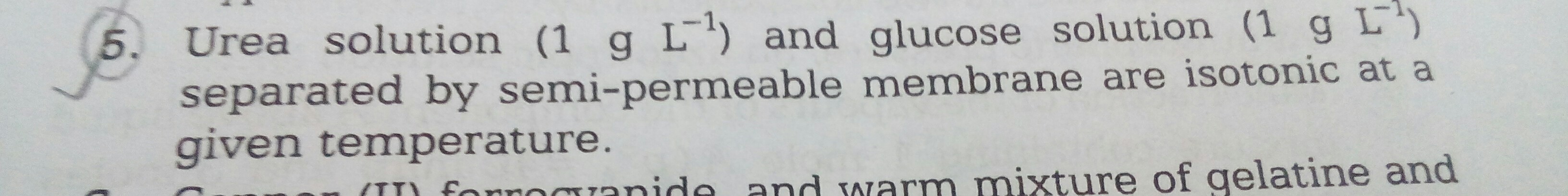
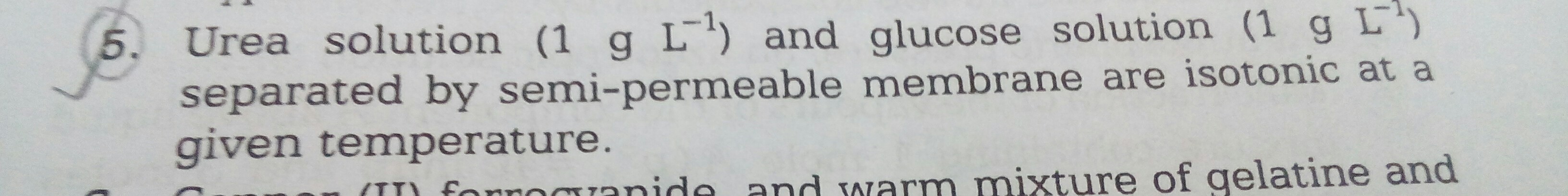
Π = (n/v)RT
Π = Osmotic pressure
C = (c/v) = concentration
n = number of moles
R = Gas constant
T = Temperature
So, Π(urea) = Π(glucose)
[n(urea) / v] RT = [n(glucose) / v]RT
Mass(urea) / molecular mass(urea) = Mass(glucose) / molecular mass(glucose)
1/60 = 1/180
n(urea) = 0.0166 moles in 1 L
n(glucose) = 0.0055 moles in 1 L
So the staement is false.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
