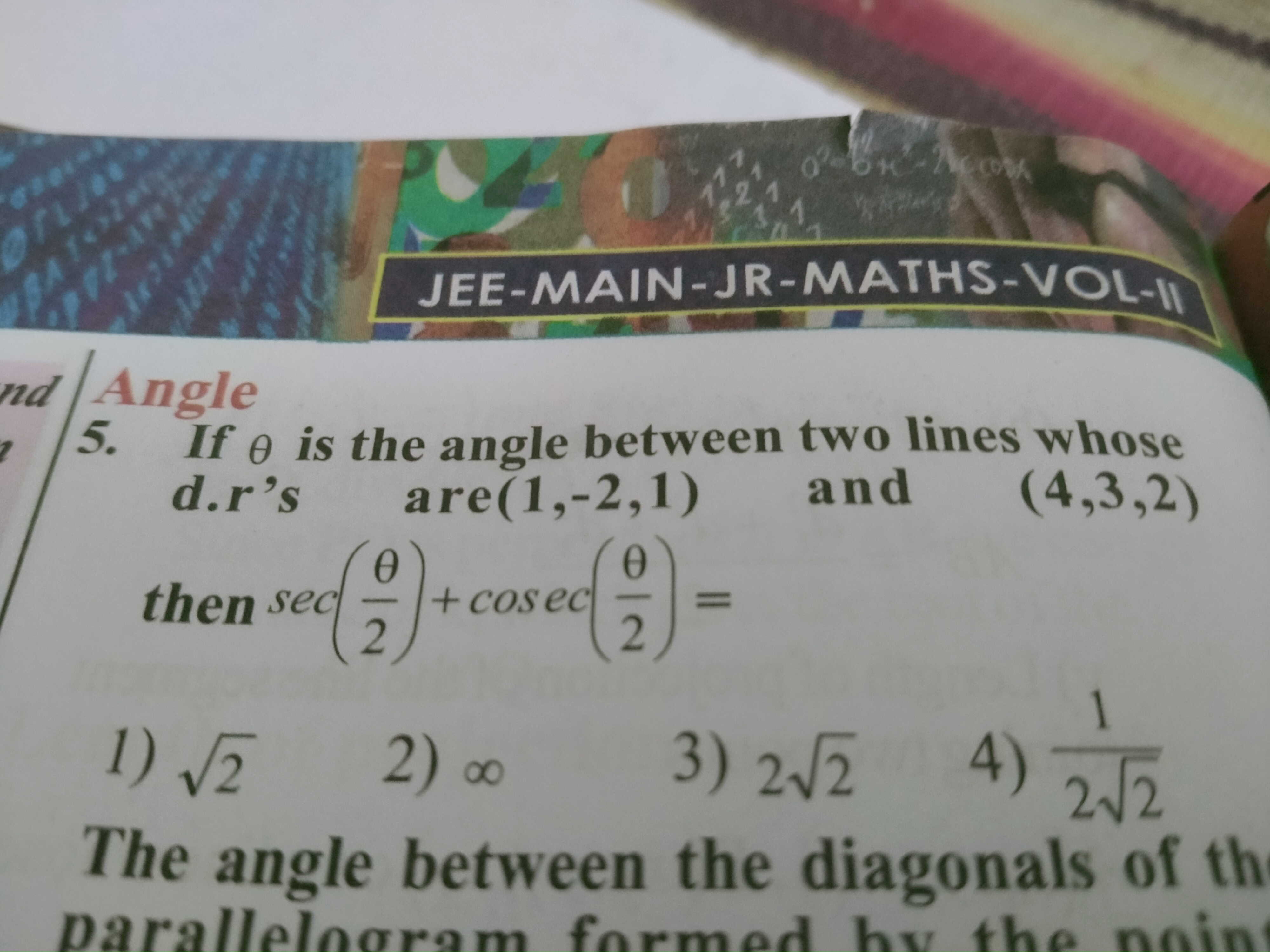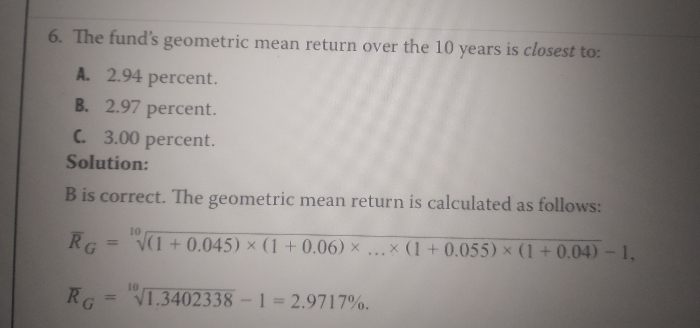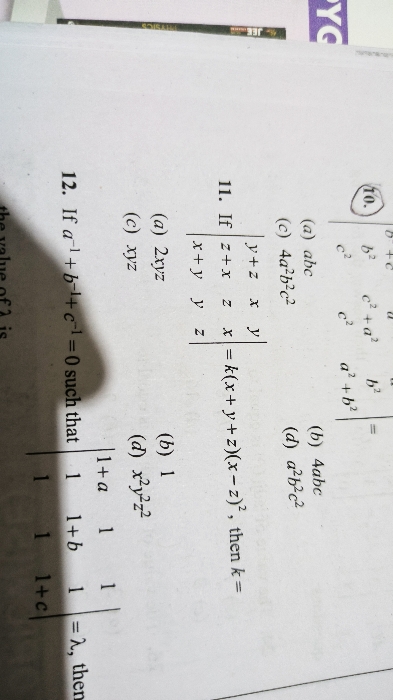JEE Main Questions and Answers
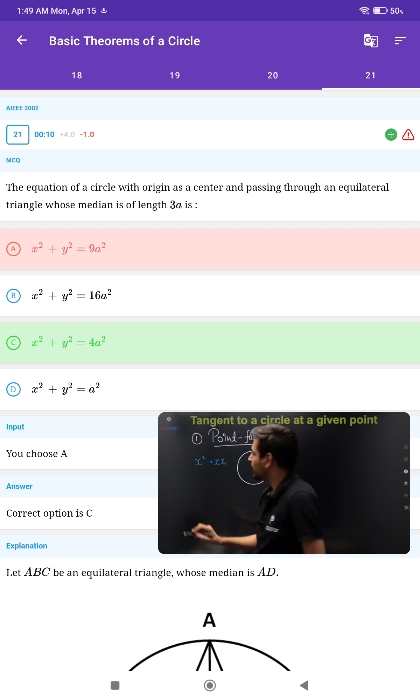
JEE Main - Maths
Asked by veerababukoppisettiveerababu3 | 17 Apr, 2024, 05:49: PM
JEE Main - Maths
Asked by usakoyalshailesh | 15 Apr, 2024, 09:48: AM
JEE Main - Maths
Asked by sharmalalita847 | 14 Apr, 2024, 09:21: PM
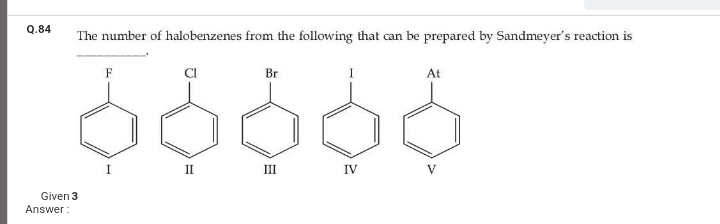
JEE Main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE Main - Maths
Asked by sarthakshukla7275 | 14 Apr, 2024, 01:29: PM
JEE Main - Maths
Asked by ashwinskrishna2006 | 14 Apr, 2024, 11:58: AM