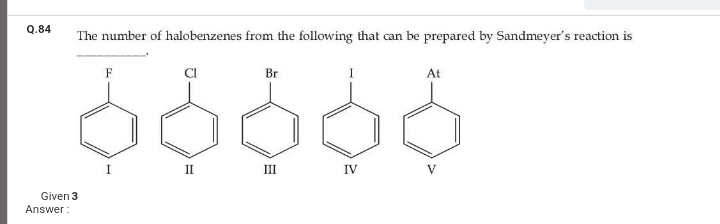
JEE Class main Answered
Is fluorobenzene or chlorobenzene more reactive towards SNAr ( Substitution Aromayic Nucleophilic Reaction) ? I heard it is fluorobenzene but shouldn't it be chlorobenzene because chlorine deactivates the benzene ring more than fluorine?
Asked by sumayiah2000 | 25 Apr, 2019, 01:53: PM
In Substitution aromatic nucleophilic reaction, A negatively charged complex(Meisenheimer Complex) is formed.This negatively charged complex will be more stable in fluorobenzene as compared to chlorobenzene because of high -I effect of Fluorine. So due to stablisation of product intermediate reaction fluorobenzene is more reactive than chlorobenzene.
Answered by Ravi | 26 Apr, 2019, 02:59: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 01:21: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 09:44: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE main - Chemistry
Asked by ruchisharmatbn | 06 Apr, 2024, 08:42: AM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 11:27: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by syamalanandini49 | 19 Mar, 2024, 11:58: AM