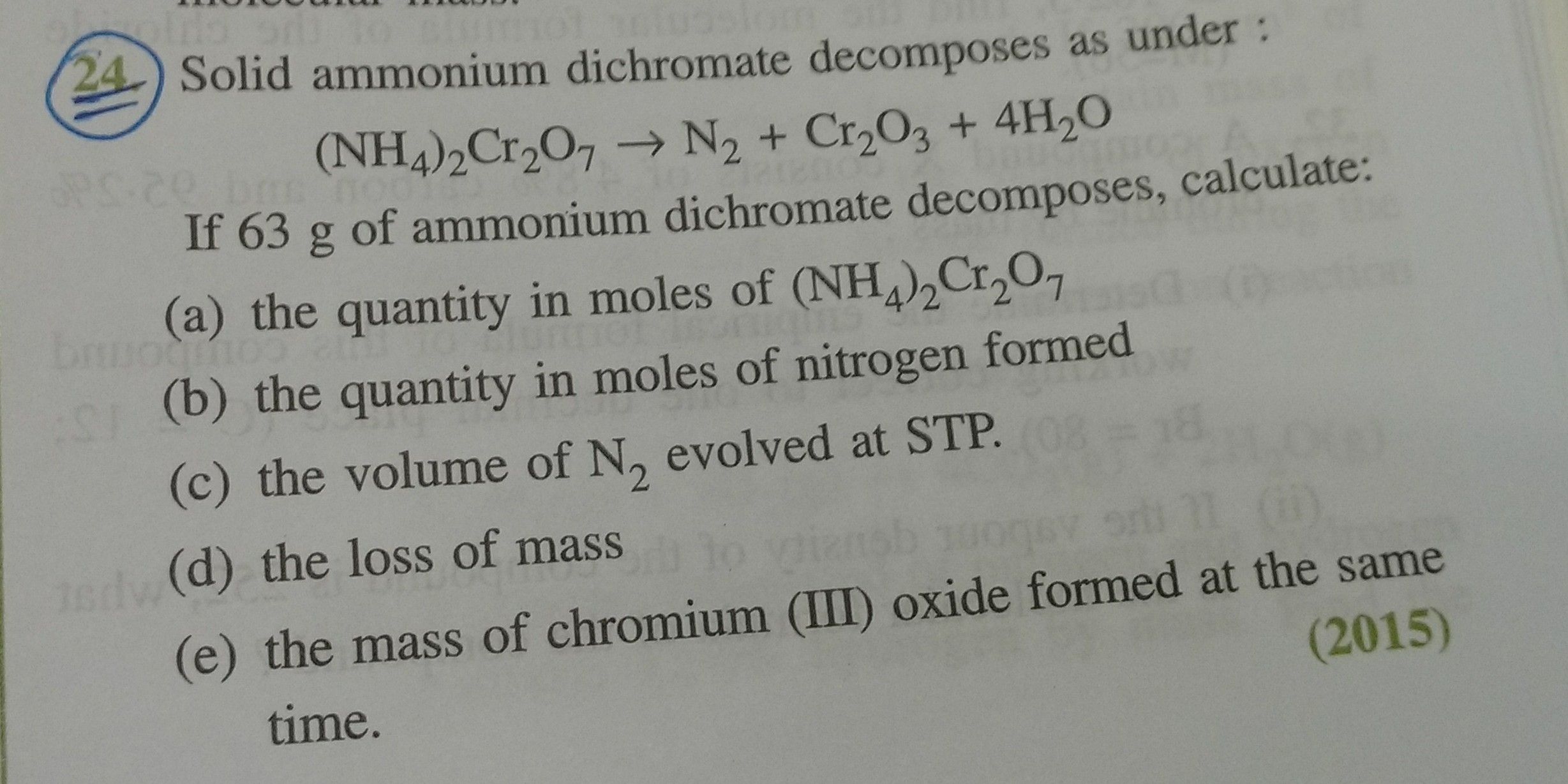ICSE Class 10 Answered
in case of relative molecular mass of a compound, should we express it in terms of grams? many a times in the questions it is expressed like that and many a times not.
Asked by sankar.goswami | 17 Feb, 2016, 07:21: PM
The relative molecular mass Mr, of a compound is the sum of the relative atomic masses (atomic weights) of the atomic species as given in the chemical formula. The mass of a molecule is compared with that of an atom of carbon-12.
Relative molecular mass is a dimensionless quantity, it has no unit. This is because they have cancelled in their calculation. A relative molecular mass can be calculated easily by adding together the relative atomic masses of the constituent atoms. For example, In ethanol, CH3CH2OH, it has a relative atomioc mass Mr = 46.
Relative molecular mass is a dimensionless quantity, it has no unit. This is because they have cancelled in their calculation. A relative molecular mass can be calculated easily by adding together the relative atomic masses of the constituent atoms. For example, In ethanol, CH3CH2OH, it has a relative atomioc mass Mr = 46.
Answered by Hanisha Vyas | 18 Feb, 2016, 11:23: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by maybe.kushagra | 25 Jan, 2024, 03:12: AM
ICSE 10 - Chemistry
Asked by srinu2020.ravipati | 16 Sep, 2020, 03:33: PM
ICSE 10 - Chemistry
Asked by Gurdev71 | 24 Jun, 2020, 12:41: PM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 11:22: AM
ICSE 10 - Chemistry
Asked by aashimegh | 17 Aug, 2019, 02:24: PM
ICSE 10 - Chemistry
Asked by aashimegh | 03 Aug, 2019, 11:50: AM
ICSE 10 - Chemistry
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
ICSE 10 - Chemistry
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
ICSE 10 - Chemistry
Asked by yajay0441 | 27 Aug, 2018, 03:15: PM