NEET Class neet Answered
In AO2 the metal ions form of face centred cubic lattice the oxide ions occupy the tetrahedral sites the metal ions occupying the faces and corners of the unit cells the total number of metal ions and oxide ions per unit cell is
Ans = 12 ??
Asked by ntg432000 | 13 Feb, 2019, 06:33: PM
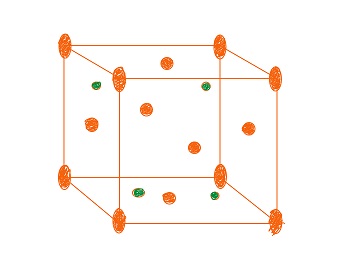 Here the balls coloured with red are The Metal Ions.
Here the balls coloured with red are The Metal Ions.So, the number of Metal atoms per unit cell = 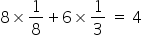
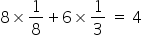
Number of Oxide ions = 8 (4 in each tertahetdral site).
So, Total No. of ions = 12 per unit cell.
Eg. CaF2 , BaF2 Structures
Answered by Sumit Chakrapani | 15 Feb, 2019, 02:02: AM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM
NEET neet - Chemistry
Asked by ghousiakaneez | 03 Apr, 2024, 12:55: PM




















