NEET Class neet Answered
In a NaCl type crystal distance between Na+ and Cl- ion is 2.814 A and the density of solid is 2.167 g cm-3 then find out yhe value of Avogadro's constant.
Asked by harinderamit1234 | 02 Jan, 2020, 01:23: AM
Given:
Z for NaCl = 4
Density = 2.167 g cm-3
Molar mass of NaCl = 58.5
NaCl: FCC structure = 2(rNa + RCl) = a
a = 2.814 × 2
a =5.682 Å
We know,
1 Angstrom = 100 pm
1 pm = 10−10 cm
a =568.2 pm
a =568.2 × 10−10 cm
We have,
Density
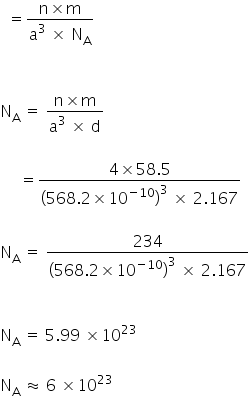
Avogadro's constant = 6.0 × 1023
Answered by Varsha | 02 Jan, 2020, 01:11: PM
Concept Videos
NEET neet - Chemistry
Asked by ashvinipawar2512005 | 31 May, 2022, 11:54: AM
NEET neet - Chemistry
Asked by ravihr1974 | 19 Apr, 2020, 03:02: PM
NEET neet - Chemistry
Asked by harinderamit1234 | 02 Jan, 2020, 01:23: AM






