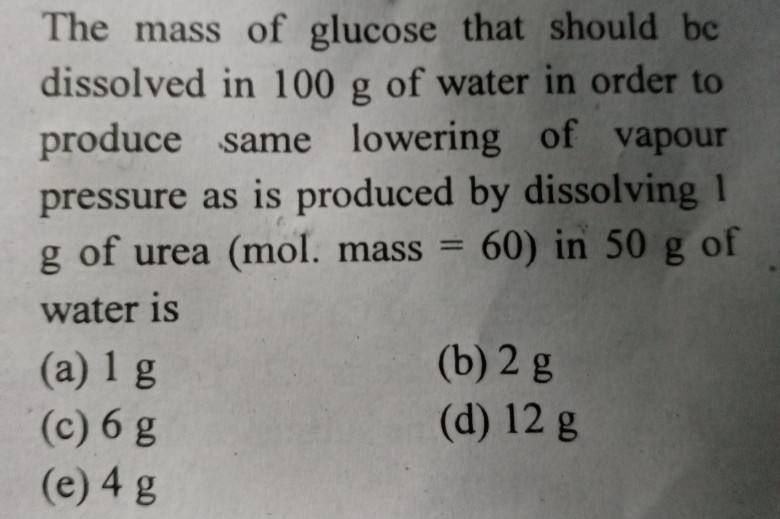NEET Class neet Answered
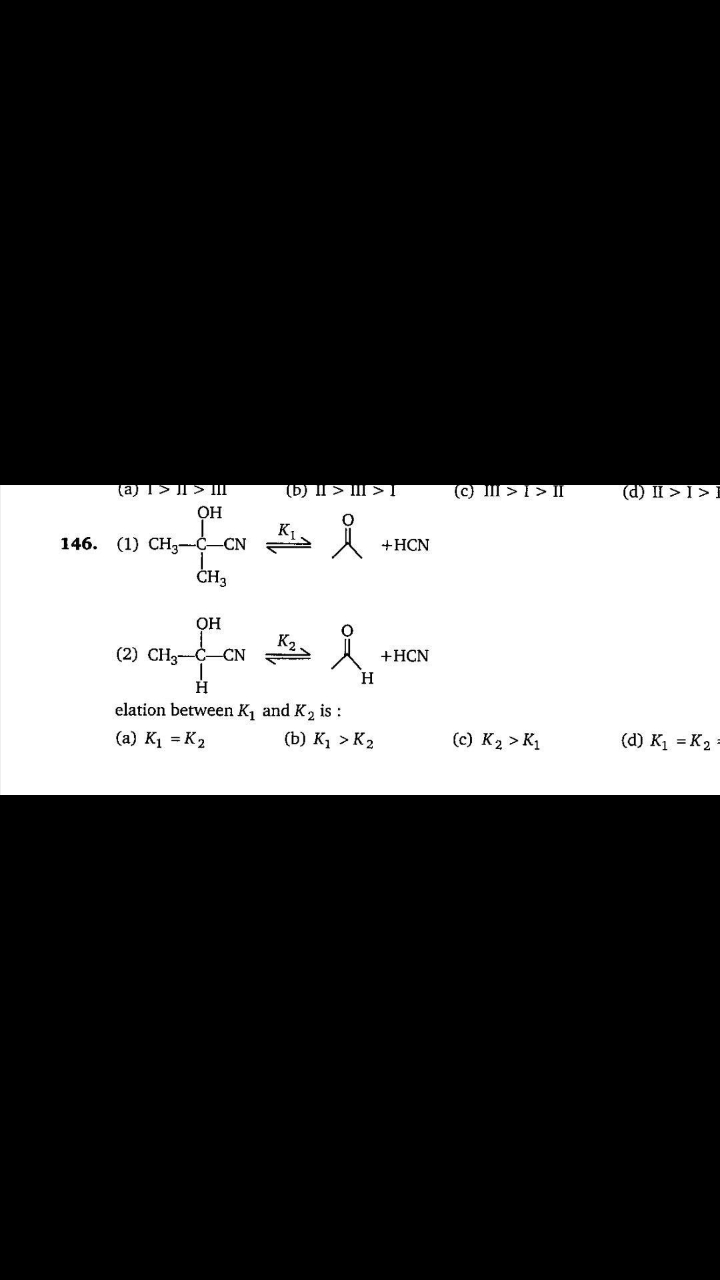
In 100 g of naphthalene , 2.423 g of S was dissolved . Melting point of naphthalene is 80.1 celcius .Tf is 661 celcius . Lf is 35.7 cal/g of naphthalene . Molecular formula of sulphur added is
(a)S2
(b)S4
(c)S6
(d)S8
Asked by Balbir | 17 Jul, 2019, 04:48: PM
Given:
Melting point of napthalene Tf = 80.1ºC = 353K
Lf = 35.7 cal/g
WA = 0.661
WB = 2.423g
Formula used:
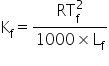
And the formula for depression in freezing point,
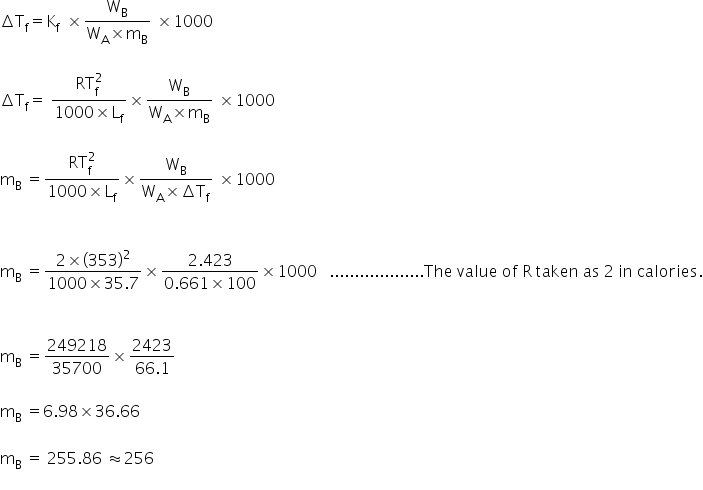
Since, the atomic weight of sulphur is 32 and its molecular mass is 256, therefore number of sulphur atoms associated to form a single molecule is 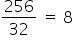
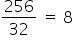
Hence the molecular formula of sulphur is S8
Answered by Ramandeep | 22 Jul, 2019, 05:27: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by prakriti12oct | 28 Apr, 2020, 01:31: AM