ICSE Class 10 Answered
If specific heat capacity of solid = 500J perkg pe r°C
Calculate the specific latent heat of fusion of substance
Asked by lovemaan5500 | 15 Mar, 2018, 07:32: AM
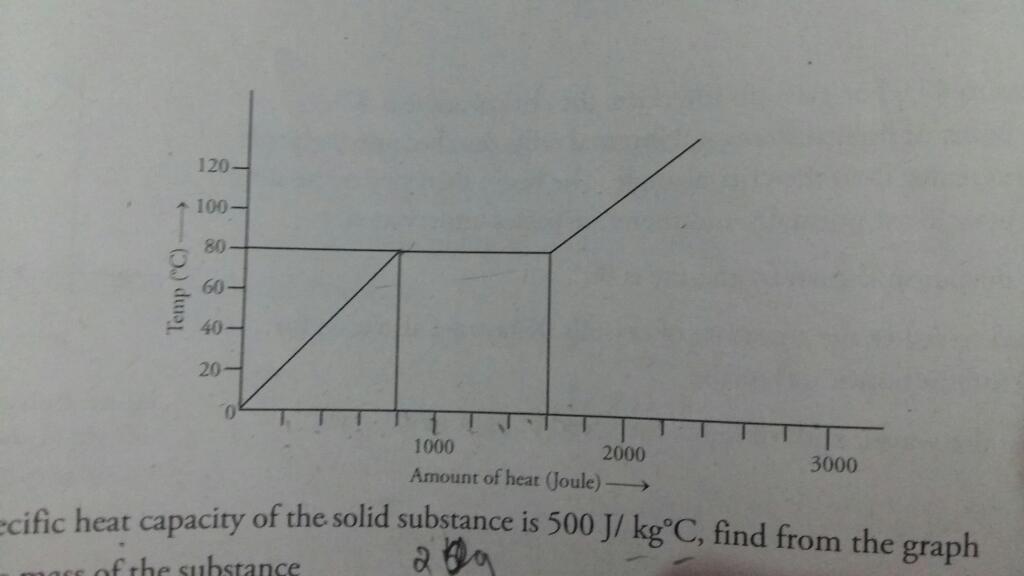
As per the given graph, 800 J of thermal energy is used to raise the temperature of the given substance from 0ºC to 80ºC. Mass of the substance is calculated from the give specific heat value as given below
m×Cp×ΔT = m×500×80 = 800; where m is mass, Cp is specific heat and ΔT is increase in temperature;
As per the graph, 800 J of thermal energy is used to melt completely the substance at constant temperature 80ºC.
Hence latent heat of fusion of the substance L is calculated as given below
m×L = 0.02×L = 800 J; we get L = 40 kJ/kg
we get m = 0.02 kg;
Answered by Thiyagarajan K | 15 Mar, 2018, 11:54: AM
Application Videos
Concept Videos
ICSE 10 - Physics
Asked by anubhutiupadhaya | 04 Mar, 2024, 01:04: PM
ICSE 10 - Physics
Asked by navycuber2738 | 29 Feb, 2024, 02:14: PM
ICSE 10 - Physics
Asked by vijayprabath7 | 28 Jan, 2024, 04:41: PM
ICSE 10 - Physics
Asked by foodonly742 | 02 Jan, 2024, 11:06: AM
ICSE 10 - Physics
Asked by krishnathakurt139 | 06 Dec, 2023, 09:23: PM
ICSE 10 - Physics
Asked by ayanpal713143 | 27 Nov, 2023, 09:32: PM
ICSE 10 - Physics
Asked by imunilu786 | 29 Oct, 2023, 02:35: PM
ICSE 10 - Physics
Asked by praggya.srivastava.g1972 | 13 Oct, 2023, 10:51: AM
ICSE 10 - Physics
Asked by praggya.srivastava.g1972 | 11 Sep, 2023, 08:48: PM
ICSE 10 - Physics
Asked by praggya.srivastava.g1972 | 10 Sep, 2023, 10:52: PM