CBSE Class 11-science Answered
I get confused in molecular mass,molar mass ,and formula unit mass can you please explain this topic with a day to day life example???
Asked by Agil | 16 Jun, 2018, 08:18: PM
Molecular mass: It is the number equal to the sum of the atomic masses of the atoms in the molecule.
Example: Molecular mass of NaCl is
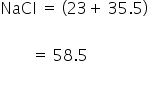
Molar mass:
Molar mass is numerically same as molecular mass but the unit is different.
The unit is gm/mole
Formula unit mass:
Formula unit mass of the substance is the sum of the atomic masses of the atoms in the formula unit of the molecule.
Answered by Varsha | 17 Jun, 2018, 01:39: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM
CBSE 11-science - Chemistry
Asked by saijagdale9 | 19 Jun, 2023, 02:34: PM
CBSE 11-science - Chemistry
Asked by kdimple765 | 17 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:23: PM




