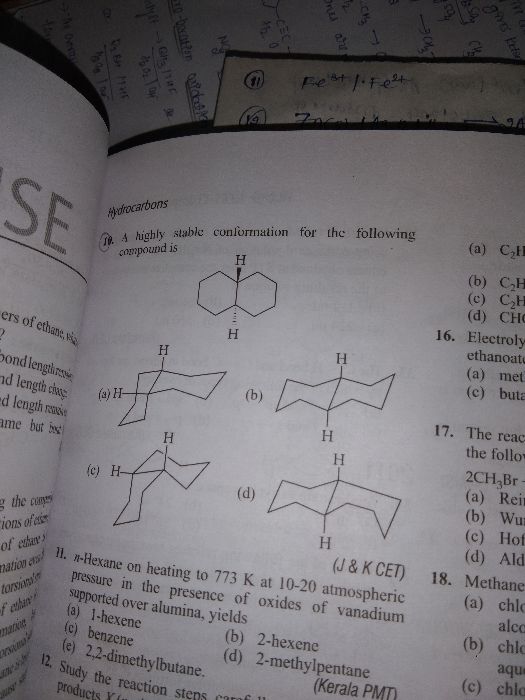
CBSE Class 11-science Answered
How will iso butane have weak intermolecular forces and how it has an higher boiling point than n butane if n butane's intermolecular forces are stronger than that of iso butane?
Asked by Arun Iyer | 26 Jan, 2011, 12:00: AM
Dear Student
I think there is a small confusion.
Butane has a higher boiling point because the dispersion forces are greater. The molecules are longer (and so set up bigger temporary dipoles) and can lie closer together than the shorter, fatter 2-methylpropane molecules.
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 27 Jan, 2011, 10:09: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM