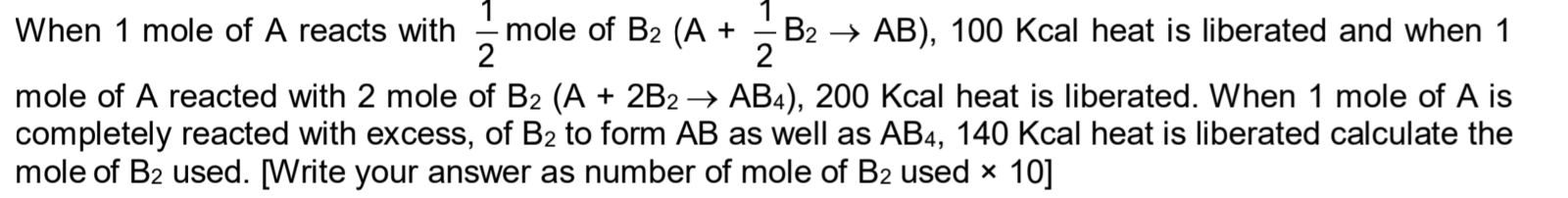CBSE Class 11-science Answered
how to solve this ?

Asked by arijitbhkt | 12 Sep, 2018, 09:26: AM
The reaction equations are as :
(NH4)2SO4 + 2NaOH → 2NH3 + Na2SO4 + 2H2O ------ (1)
2NaOH + H2SO4 → Na2SO4 + 2H2O ------ (2)
Now for 5 ml 0.2 N H2SO4,
0.2 N H2SO4 = 0.1 M H2SO4
No. of moles of H2SO4 = molarity × Volume in litre
= 0.1 × 0.005
No. of moles of H2SO4 = 0.0005 mol
From reaction (2)
As 1 mole of H2SO4 requires 2 mole of NaOH
Therefore 0.0005 moles of H2SO4 will require 0.001 mole of NaOH
25 ml solution is taken for titration, which is 1/10 th of the original solution.
Therefore, no. of moles of NaOH remained after the reaction with is 0.001×10 = 0.01 mole
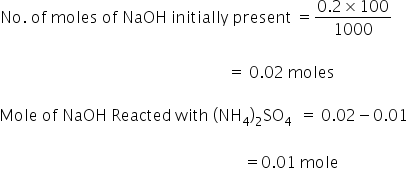
From reaction (1) 2 moles of NaOH reacts with 1 mole of (NH4)2SO4
Therefore 0.01 mole of NaOH will react with 0.005 moles of (NH4)2SO4
We have,

Percentage purity of (NH4)2SO4 is 94.28%
Answered by Varsha | 12 Sep, 2018, 02:03: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 10:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 03:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 10:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 03:58: PM
CBSE 11-science - Chemistry
Asked by pb_ckt | 19 May, 2019, 11:56: PM