CBSE Class 12-science Answered
How to calculate..
Asked by sashank2 | 19 Mar, 2010, 10:55: PM
The question is a little unclear. I am answering it according to what I have understood for it.
In general, in a compound see the central atom. Draw its lewis structure.Check its bonding and then you can check whether is there any unpaired electron in it or not.
For example:
In NO2, the structure is:
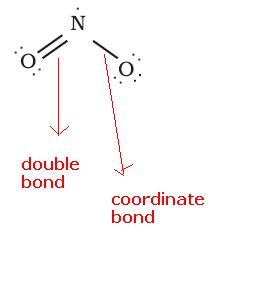
See here N has 5 valence electrons, with 2 electrons it forms a double bond, 2 electrons it donates for sharing as a coordinate bond. So, it is left one electron. So, it is an odd electron speices.
If you want to ask for any other type do ask again.
Answered by | 20 Mar, 2010, 04:21: PM
CBSE 12-science - Chemistry
Asked by Drona | 11 Jan, 2013, 12:56: PM
CBSE 12-science - Chemistry
Asked by | 06 Jan, 2013, 02:50: PM
CBSE 12-science - Chemistry
Asked by Abhijith C | 06 Jan, 2013, 04:52: AM
CBSE 12-science - Chemistry
Asked by Akhil Mohan | 04 Jan, 2013, 08:21: PM
CBSE 12-science - Chemistry
Asked by zrajani | 31 Dec, 2012, 08:26: PM
CBSE 12-science - Chemistry
Asked by Yatin gupta | 31 Dec, 2012, 12:59: AM
CBSE 12-science - Chemistry
Asked by Yatin gupta | 31 Dec, 2012, 12:58: AM
CBSE 12-science - Chemistry
Asked by Akash Sundi | 28 Dec, 2012, 05:10: PM
CBSE 12-science - Chemistry
Asked by Yatin gupta | 24 Dec, 2012, 05:54: PM
CBSE 12-science - Chemistry
Asked by Debmalya Ghosh | 21 Dec, 2012, 01:59: PM

