CBSE Class 12-science Answered
How much coilomb required for oxidation of mole of H2O to O2
Asked by j4jaigupta1998 | 09 Jun, 2015, 10:17: AM
Given:
We have 1 mol of H2O
1 mol of H2O will give one atom of O
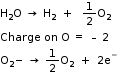
Electricity required for the oxidation of 1 mole of H2O to O2 = n F
(Here, n= 2)
= 2 × 96487 Coulombs= 192974 Coulombs
= 1.93 × 105 Coulombs
Answered by Hanisha Vyas | 09 Jun, 2015, 12:27: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 09:14: PM
CBSE 12-science - Chemistry
Asked by sy985326 | 21 Feb, 2024, 04:23: AM
CBSE 12-science - Chemistry
Asked by rishitadekaraja123 | 07 Feb, 2024, 08:41: AM
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 08:39: PM
CBSE 12-science - Chemistry
Asked by skmdsajid04 | 14 Jan, 2024, 09:23: AM
CBSE 12-science - Chemistry
Asked by samskruthikrishn | 12 Jan, 2024, 10:11: AM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by 2507king2006 | 03 Oct, 2023, 07:12: AM
CBSE 12-science - Chemistry
Asked by keerthana.d.cst.2022 | 22 Aug, 2023, 08:18: PM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 08:51: PM







