How many optically active stereoisomers are possible for butan-2,3-diol?
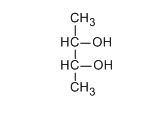
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
