JEE Class main Answered
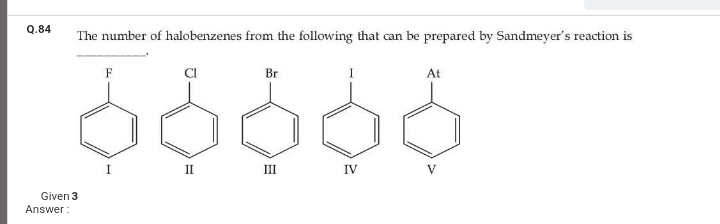
how many moles of pbcl2 formed

Asked by ashutosharnold1998 | 14 Apr, 2020, 12:01: PM
The moles of lead(II) chloride formed can be calculated as:
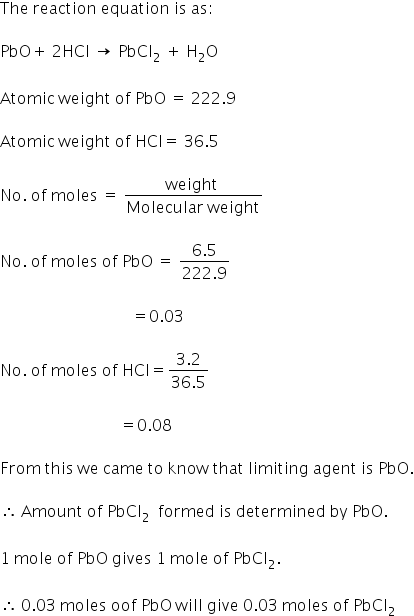
Answered by Varsha | 14 Apr, 2020, 07:33: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 01:21: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
JEE main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE main - Chemistry
Asked by ruchisharmatbn | 06 Apr, 2024, 08:42: AM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 11:27: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 08:48: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by syamalanandini49 | 19 Mar, 2024, 11:58: AM