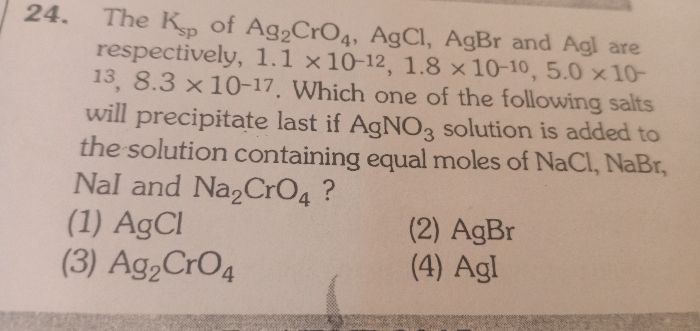CBSE Class 11-science Answered
How is solubility different from solubility Product?
Asked by Topperlearning User | 30 Apr, 2015, 02:30: PM
The solubility of a solute is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature.
Unlike the solubility of a substance, the solubility product is independent of what else is dissolved in solution. The solubility of a substance can be calculated from its solubility product.
Answered by | 30 Apr, 2015, 04:30: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 02:33: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 02:32: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 18 Apr, 2020, 02:21: PM
CBSE 11-science - Chemistry
Asked by Anish | 23 Aug, 2019, 01:48: AM
CBSE 11-science - Chemistry
Asked by jhajuhi19 | 12 Jun, 2019, 07:23: PM
CBSE 11-science - Chemistry
Asked by Prakash | 28 Jun, 2018, 06:09: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 06:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 May, 2015, 03:13: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 02:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 05:24: PM