CBSE Class 11-science Answered
how does hybridisation take place in :-
(a) C2H2
(B)PCl5
Asked by rajenderdagar123 | 08 Jan, 2017, 06:42: PM
Structure of C2H2:
- The ground state configuration of ‘C’ being 1s2 2s2 2px1 2py1 has only two unassociated electrons. Carbon makes four bonds because its valency is four.
- For this, 4 unpaired electrons are required. Hence, it promotes its 2s electron to the empty 2pz orbital in the excited state.
- So, the excited state electronic configuration of carbon is 1s2 2s1 2px1 2py1 2pz1. Each carbon atom undergoes ‘sp’ hybridisation by using 2s and 2p orbitals in the excited state to give two half-filled ‘sp’ orbitals, which are arranged linearly.
Formation of PCl5 (sp3d Hybridisation)
- The ground state and the excited state outer electronic configurations of phosphorus (Z = 15) are given below:
- The formation of five half-filled sp3d hybrid orbitals involves the intermixing of one 3s, three 3p and one 3d orbitals during excitation.
- They are then organised in a trigonal bipyramidal symmetry. While three orbitals are organised in trigonal planar symmetry, the remaining two are kept perpendicularly above and below this plane.
- These half-filled sp3d orbitals help phosphorus in forming five σsp3d-p bonds with chlorine atoms. Each chlorine atom makes use of half-filled 3pz orbital for the bond formation.
- The PCl5 molecule has a trigonal bipyramidal shape with 120° and 90° of ∠Cl–P–Cl bond angles.
-
Answered by Vaibhav Chavan | 09 Jan, 2017, 11:24: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


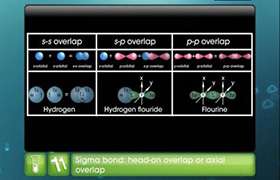
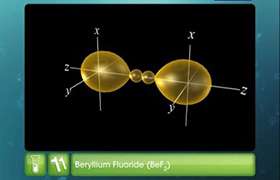
 on the basis of hybridisation
on the basis of hybridisation